User login
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
Operative vaginal delivery: 10 components of success
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
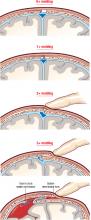
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
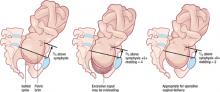
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
Operative vaginal delivery is a dying art. National databases in the United States and elsewhere have shown this trend for decades.1 Women no longer can be reliably predicted to prefer operative vaginal delivery over cesarean section, and providers caring for delivering mothers (and their families) should not assume that they do. Nor does the 20th century paradigm of operative vaginal delivery as the accepted “next step” between spontaneous vaginal delivery and cesarean section hold up, given the decreased maternal and neonatal morbidity and mortality associated with modern techniques of cesarean section. Nevertheless, operative vaginal delivery remains a viable option in some cases.
This article—based on personal opinion and experience, as well as published data whenever possible—describes 10 selected aspects of operative vaginal delivery, offering recommendations for each.
1. Consider obstetric history
How a woman fared in previous deliveries has a bearing on the current delivery. For example, if she has a history of persistent occiput posterior position, as in the case described on page 56, she may have an anthropoid pelvis, placing her at increased risk for another malposition.1 In such cases, the patient should be counseled about the potential for operative vaginal delivery, and the risks and benefits should be discussed prenatally.
A history of obesity, excessive weight gain, and glucose intolerance should be considered warning signs of a large-for-gestational-age infant.
2. Ensure adequate informed consent
Patients should be informed of the risks of any procedure they are offered, and operative vaginal delivery—like any operative procedure—has definite risks.
It is unbalanced to mention only the perceived benefits of a procedure and to avoid the discomfort of discussing the potential significant fetal and maternal injury that may result from a procedure. It is far better for the patient and her family to learn—before an adverse outcome occurs—that forceps delivery sometimes leads to maternal and fetal lacerations, and that operative vaginal delivery can be associated with an increased risk for shoulder dystocia in some circumstances.
The best way to educate patients about operative vaginal delivery is during prenatal care. I recommend a written informed consent document similar to the one used for cesarean section. If such a form is not signed during the course of office prenatal care, it should be offered upon admission for delivery.
In some cases, operative vaginal delivery may be safer than cesarean
Operative vaginal delivery clearly increases the risk of neonatal intracranial bleeds when compared with normal spontaneous vaginal delivery or elective cesarean section.2 However, a patient should understand that cesarean section carries a risk of neonatal intracranial hemorrhage similar to that of operative vaginal delivery once a woman has labored to complete dilation and pushed for some time.2 In fact, a baby with a well-engaged head can experience significant increases in intracranial pressure during cesarean delivery when concerted efforts have to be used to deliver a deeply engaged fetal head out of a hysterotomy incision. Such maneuvering can also injure the fetal neck and brachial plexus.
3. The abdominal examination is critical
Examination of the maternal abdomen helps to confirm the fetal lie and presentation and may give an idea of the position of the fetal back in relation to the uterine midline. If the fetal back cannot be felt or is palpated far laterally, the fetus may be in an occiput posterior or transverse position. Often this knowledge helps the examiner make sense of an otherwise difficult vaginal examination.
Estimate fetal weight
Fetal weight estimations from a careful abdominal examination can be as accurate as ultrasonographic evaluation.3 It is strongly recommended that fetal weight be estimated and considered in context with maternal diabetes, obesity, excessive weight gain, and previous ultrasound examinations before operative vaginal delivery is undertaken.
Is the fetal head engaged?
The average term (3,200 g) fetus has a basovertical head diameter of approximately 9 to 10 cm,4,5 and the average adult finger has a diameter of 2 cm (one fifth of the head). Using this information, an estimate of how many “fifths” of the fetal head are above the pelvic brim can be made by evaluating how many fingerbreadths of fetal head can be palpated above the symphysis pubis on abdominal examination.
Crichton4 described this method in 1974, and it is an extremely useful and underutilized technique, in my opinion. He stated that no more than two fifths (2 fingerbreadths) of an unmolded fetal head should be palpated abdominally once the occiput is felt at the ischial spines. If three fifths or more of the fetal head is still palpable above the pubic symphysis, regardless of whether there is bone palpated at or below 0 station on vaginal examination, consider the head unengaged and avoid operative delivery.
It is quite possible to feel the fetal skull bone below the ischial spines and still have an unengaged head.5 This is due to molding of the head and elongation of the basovertical diameter. When this occurs, the widest diameter of the fetal skull remains above the plain of the pelvic brim (unengaged), even though the lowermost point is felt below the spines on vaginal examination. A graphic example of such an elongated basovertical diameter can be seen in the so-called cone-head baby.
At examination, fetal head should be in occiput anterior position
In order to best use the abdominal examination to assess the amount of fetal head above the pelvic brim, the fetal head must be in an occiput anterior position. This is because the occiput is sometimes difficult to palpate in a posterior or transverse position, and the obstetrician may incorrectly assume full engagement. This further underscores the importance of a careful maternal abdominal examination and the location of the fetal spine.
Abdominal examination is more informative than vaginal examination
Knight and colleagues6 studied the relative value of abdominal and vaginal examinations in the determination of fetal head engagement. They examined the records of 104 women who had been evaluated by both methods prior to attempted operative vaginal delivery. Successful vaginal delivery was correctly predicted using abdominal criteria (94%) more often than using vaginal criteria (80%) (P<.01).
E.D., a 32-year-old gravida 4 para 3, presents at 39 weeks’ gestation with spontaneous rupture of membranes in early labor. Her 3 deliveries thus far have all been vaginal, with the infants ranging in weight from 3,700 to 3,900 g. Two of these infants were delivered with vacuum extraction because of occiput posterior position and a prolonged second stage.
E.D.’s prenatal course has been relatively uncomplicated except for a 43-lb weight gain (she weighs 240 lb) and a borderline 1-hour glucose challenge test. She also had 1 abnormal value on a 3-hour glucose tolerance test. Her prenatal pelvic examination was documented as “adequate.”
In early stages, all appears normal
On admission, E.D. is dilated 4 cm with 70% effacement and a cephalic presentation at -2 station. Electronic fetal monitoring is reassuring, and she is contracting regularly every 6 minutes, with moderate pain. The physician on call instructs the nurse to start oxytocin if there is no progress in 2 hours, and to call anesthesia to give an epidural if the patient requests it. E.D. asks for, and is given, an epidural 2 hours later, when her cervix is dilated 5 cm.
The next morning, a different physician examines her and reports a rim of cervix remaining, with the fetal head at 0 to +1 station. He asks E.D. to push, and the rim is reduced over the infant’s head. The patient is instructed to continue pushing with contractions. The physician writes the admission (and only predelivery) note: “32 yr old G4P3, term, SROM, good FHTs, good progress, complete, 1+ station, clear fluid. Anticipate vaginal delivery.”
When progress stalls, mother tires
E.D. pushes well with adequate contractions for 2.5 hours, with minimal descent of the head and increasing caput and molding. The physician examines her again and reports that the baby is at +2 station. He also suggests the use of the vacuum extractor, because the patient is becoming exhausted and the baby is “quite big.” The obstetrician appears somewhat hesitant when applying the vacuum and remarks to the nurse that he “thinks the baby is in a left occiput anterior position” but is not “100% sure.”
When vacuum fails, a switch to forceps
After 2 attempts with the vacuum extractor, during which there are 2 “pop-offs,” the physician asks for Simpson forceps, adding that he thinks the baby is now in right occiput posterior position and he needs to “get a better grip on the baby’s head.” The forceps are applied with some difficulty, necessitating 2 reapplications.
After 5 contractions (and 6 pulling efforts), a baby boy is delivered. Because of a delay in delivery of the shoulders after delivery of the head, the physician places the patient in McRoberts position and has a nurse apply suprapubic pressure, and no further difficulties are encountered.
Large baby has brachial plexus injury
The infant weighs 4,200 g and has Apgar scores of 3 and 8, as well as a small laceration on his forehead, moderate flaccidity of the left arm, and an elongated head. The mother has a 4th-degree laceration that is repaired with some difficulty.
The delivery note reads: “Assisted vaginal delivery, 4,200 g male, 3 vessel cord, 600 cc estimated blood loss, 4th-degree laceration repaired in layers.” E.D. ultimately requires 2 U of blood on postpartum day 2 for symptomatic anemia.
Mother and baby are discharged on postpartum day 4 in stable condition. The infant has a brachial plexus injury that resolves within 6 weeks.
Lessons learned
Among the mistakes the obstetrician made in this case are a failure to take the obstetric history into account, omission of a comprehensive abdominal exam, ignoring signs of a large baby, and lack of a plan for emergent cesarean section.
4. Keep molding in mind
Some (up to +2) occipito-parietal molding may be normal in the late stages of delivery (ie, the occipital bone slips under the 2 parietal bones, but can be easily reduced), but severe parieto-parieto molding is never normal and should be interpreted as a sign of relative or absolute cephalopelvic disproportion. FIGURE 1 shows a classification system for molding.
FIGURE 1 How to characterize the degree of molding
Excessive molding may lead to tears in dura and underlying vessels.
Traction plus severe molding may increase the risk of intracranial injury
The most frequent causes of molding are asynclitism and deflexion of the head, commonly seen in occiput posterior and transverse positions. Correction of the asynclitism and malposition may correct the molding and allow safe vaginal delivery. Traction on a head with severe molding may increase the risk of intracranial injury.
Using maximum likelihood logistic regression analyses, Knight and colleagues6 demonstrated that the factor of greatest importance in determining whether a case would be allocated to engaged versus unengaged groups was molding (odds ratio 2.17; 95% confidence intervals 0.75–6.27). The authors concluded that when abdominal and vaginal assessments produce different findings, the major factor responsible is molding. They noted that data derived from vaginal examination alone may be misleading when molding is present.
The rule of 3’s
With the fetus in an occiput anterior position, determine the number of fifths of the fetal head that can be palpated above the pelvic brim abdominally, add it to the degree of molding palpated vaginally, and avoid operative vaginal delivery if the sum is 3 or higher (FIGURE 2).7
FIGURE 2 Abdominopelvic assessment using the rule of 3’s
If the sum of the number of fifths of the fetal head palpated above the pelvic brim abdominally and the degree of molding palpated vaginally equals or exceeds 3, operative vaginal delivery is unlikely to be successful.For example, if two fifths (~4 cm) of the fetal head is palpated above the maternal pubic symphysis, and there is already +1 of parieto-parieto molding, significant cephalopelvic disproportion is likely and operative vaginal delivery will probably fail, with an increased risk of fetal and maternal damage. Obviously, if three fifths or more of the fetal head is palpated abdominally, the head is not engaged and operative vaginal delivery is contraindicated, regardless of whether the scalp is felt at or below the ischial spines.
Knowledge of fetal head diameters is useful
Using measurements of fetal head diameters (FIGURE 3), it is easy to see why a vertex presentation in an occiput anterior position (presenting diameter=suboccipitobregmatic diameter=9.5 cm) will deliver more easily than a baby in a deflexed occiput posterior position (presenting diameter=occipitofrontal diameter ≥ 11.5 cm). The presenting diameter of a brow presentation will never negotiate a normally proportioned female pelvis, whereas that of a mentum anterior face presentation is clearly adequate for a vaginal delivery if all other factors are favorable.
FIGURE 3 Know the basic term fetal head diameters
Depending on the presentation, the fetal head will deliver easily, as in occiput anterior position, when the presenting diameter is 9.5 cm, or with difficulty, as when the presenting diameter is 11.5 cm or more.
5. Be aware of fetal head position throughout labor
Early documentation of fetal head position during labor may help tremendously when decisions regarding the mode of delivery have to be made in a hurry. If one is aware that the head has been persistently in a deflexed occiput posterior position (or a transverse position) throughout the labor, a prolongation or arrest of descent can be explained by progressive deflexion of the head and increasing presenting diameters (or deep transverse arrest, as the case may be).
In such a case, if sudden fetal decompensation necessitates emergent delivery, operative vaginal delivery is a much less viable option than it would be with a well-flexed occiput anterior position at the same station.
When there is knowledge of a fetal malposition, cesarean section may be the wisest choice in an emergency, even if the fetal head is at an appropriate station, unless the operator has the requisite skills at operative vaginal delivery and is certain of a high chance of a successful outcome.
6. Have a valid indication
Operative vaginal delivery should not proceed without a valid indication8 that conforms to accepted guidelines.
Consider the pathology underlying the indication
For example, although maternal exhaustion is clearly a valid indication for operative vaginal delivery, it is important to examine the underlying reason for the exhaustion. A diabetic mother who is known to have a large-for-gestational-age infant, who has a prolonged active phase (8 hours) and is exhausted after 3 hours of excellent pushing with adequate contractions, may appear to have, on the surface, a valid indication—but clearly this situation calls for extreme caution. The size of the infant and the lack of progress suggest at the least the potential for cephalopelvic disproportion.
In my opinion, operative vaginal delivery for maternal exhaustion should probably be reserved for someone who has progressed at a normal rate to crowning and who simply does not have the energy to push out a normal-sized baby.
Judicious use is key
Operative vaginal delivery can be used judiciously to remedy situations that have the potential to escalate. For example, a persistent transverse fetal head position in a primigravida with a platypelloid pelvis who has pushed for 1 hour with increasing caput and molding is highly unlikely to resolve. If the fetus is an appropriate candidate for rotational forceps, and the physician has the requisite training and experience, a rotational delivery after only 1 hour is entirely appropriate to avoid potential maternal and fetal injury that could follow 3 hours of pushing.
Persistent variable decelerations are another indication of potential fetal compromise and justify judicious use of operative vaginal delivery in appropriate candidates.
7. Do not use instruments sequentially
The use of sequential operative vaginal delivery methods to complete a vaginal delivery is no longer acceptable. Significantly increased neonatal and maternal risks have been demonstrated in at least 3 well-designed studies. Data indicate that a failed operative vaginal delivery attempt,2,9,10 more than 3 hours of maternal pushing,10 and more than 3 traction episodes (regardless of ultimate success with the instrument)10 are associated with an increased risk of neonatal intracranial hemorrhage.
Because we lack a standard of care for the optimal number of traction efforts or “pop-offs” for operative vaginal delivery, I suggest that any practitioners be familiar with, and adhere to, the manufacturer’s suggested guidelines. These guidelines will usually be designed to fall on the conservative side of safety issues.
I have heard of physicians who sometimes use the vacuum extractor to bring the head down to a place where they feel more comfortable applying forceps. This practice is unacceptable. By the same token, proceeding with vacuum extraction after concluding there is too much molding or caput for forceps is untenable.
8. Have a clear endpoint and exit strategy
Resist the temptation to persist with operative vaginal delivery in the face of inadequate descent or progress. It may sometimes seem as though “just one more pull” will effect delivery, but exceeding the recommended number of attempts can lead to excessive traction and maternal or fetal damage. It can be easy to become fixated on achieving vaginal delivery, and rational thought can become clouded.
I recommend that each department establish clear and agreed-upon limits for their practitioners. To this end, there should be an appropriately cooperative atmosphere in each delivery unit that encourages the provider team to work together to prevent adverse outcomes from operative vaginal delivery. Protocols or checklists that help the nursing staff keep the physician informed of the number of traction efforts and/or pop-offs that occur will help prevent inadvertent exceeding of the limits established for that unit.
Prior to attempting an operative vaginal delivery, the obstetrician should have a clear exit strategy, and this strategy should be outlined to the patient and the nursing/ancillary staff. When the predetermined criteria are met, operative vaginal delivery should be abandoned without delay, and cesarean section should be performed expeditiously. Obviously, this requires that preparations for emergent cesarean section be made prior to use of the forceps or vacuum extractor.
The necessary anesthesia and neonatal and operating room personnel should be ready and in position at the time the operative vaginal delivery is attempted.
Never resume maternal pushing after failed forceps or vacuum
There is no place for “rest and descend” protocols or further attempts at spontaneous vaginal delivery after a failed operative vaginal delivery. Once an easy operative vaginal delivery becomes impossible, immediate cesarean section is the best option.
- A valid indication documented preoperatively
- Unambiguous knowledge of the fetal head position
- Complete dilatation of the cervix
- Confirmed engagement of the fetal head
- Station at or below +2, unless the operator is experienced and there is a justifiable reason for a midpelvic delivery
- Rule of 3’s satisfied
- A documented estimate of appropriate fetal weight and adequate maternal pelvic anatomy
- Adequate anesthesia
- Preparations in place for immediate cesarean section and resuscitation of the neonate, if needed
- An informed, willing, and cooperative patient who understands that cesarean section may be an appropriate alternative mode of delivery (depending on the circumstances)
In addition, the person intending to perform the delivery should personally examine the patient before the attempt to confirm that the prerequisites have been met. I would go so far as to state that, unless there is a high expectation of an easy operative vaginal delivery, it should not be attempted.
9. Document, document
Under ideal circumstances, the obstetrician initiates a discussion with the patient during prenatal care and mentions the possibility of vacuum extraction or forceps delivery. This discussion is documented in the prenatal chart. The note includes a statement discussing the relative risks and benefits of the alternative delivery modes, the patient’s expressed desire for a vaginal delivery, including operative vaginal delivery, and why, in the physician’s best judgment, an operative vaginal delivery is a reasonable option.
Document the events of labor and delivery
Clear, concise progress notes from nursing and obstetric care providers are extremely important. All pertinent maternal and fetal information should be addressed at each examination of the patient, and some comment on the rate of progress, threshold limits, management plan, and preparations should be included.
In my opinion, each progress note should describe maternal vital signs, adequacy of contractions, use of labor augmentation and the dose, fetal tolerance of contractions, reassuring nature of the monitoring, cervical dilation, fetal head position (if discernible), station, and any molding and caput. If maternal or fetal monitoring is inadequate with external devices, the notes should include details of the plan to improve the situation.
Include a preoperative note
I strongly recommend a preoperative note if there is time. It should clearly document the pertinent obstetric and prenatal care the patient has received, the progress of labor, the indication for operative vaginal delivery, estimated fetal weight, adequacy of the maternal pelvis for an infant of the anticipated weight, fetal head position, degree of molding, complete dilation of the cervix, station of the fetal head, and some assessment of flexion of the neck, if possible.
Once the decision to proceed has been made, I would add a statement indicating that the chances of success are high and, in your considered opinion, operative vaginal delivery is a safe and indicated option.
Write a detailed postoperative note
I suggest a dictated postoperative note for every operative vaginal delivery, successful or not. The elements included in the preoperative note should be reiterated and details of the delivery explained. The position and station of the fetal head at the time the instrument was applied (especially if this contrasts with what was stated in the preoperative note), the degree of caput and molding, the number and duration of traction efforts, progress of the fetal head with each traction effort, duration of the procedure, personnel present, and the preparations made for the delivery should all be documented. Physicians and nurses should agree on what constitutes a traction effort, to avoid conflicts in the various sets of notes.
Document postdelivery vaginal and rectal examinations, which should alert you to the presence of any retained sponges, vaginal hematomas or sulcus tears, or a previously unidentified rectovaginal fistula.
10. Handle bad outcomes with compassion
Do not avoid contact with the family in the event of a bad outcome. Rather, confront the outcome as honestly and compassionately as possible. If you correctly assessed and informed the patient and proceeded to operative vaginal delivery with her full understanding of the indication, she will have accepted a small risk of an untoward outcome. In general, if she perceives your behavior to have been professional and caring, she is much less likely to seek retribution.
The author reports no financial relationships relevant to this article.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.
1. Gardberg M, Stenwall O, Laakkonen E. Recurrent persistent occipito-posterior position in subsequent deliveries. BJOG. 2004;111:170-171.
2. Towner D, Castro MA, Eby-Wilkens E, Golbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
3. Fetal macrosomia. American College of Obstetricians and Gynecologists Practice Bulletin #22. Washington, DC: ACOG; November 2000.
4. Crichton D. A reliable method of establishing the level of the fetal head in obstetrics. S Afr Med J. 1974;48:784-787.
5. Crichton D. The accuracy and value of cephalopelvimetry. J Obstet Gynaecol Br Emp. 1962;69:366-378.
6. Knight D, Newnham JP, McKenna M, Evans S. A comparison of abdominal and vaginal examinations for diagnosis of engagement of the fetal head. Aust N Z J Obstet Gynaecol. 1993;33:154-158.
7. Philpott RH. The recognition of cephalopelvic disproportion. Clin Obstet Gynecol. 1982;9:609-624.
8. Operative vaginal delivery. American College of Obstetricians and Gynecologists Practice Bulletin #17. Washington, DC: ACOG; June 2000.
9. Gardella C, Taylor M, Benedetti T, Hitti J, Critchlow C. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol. 2001;185:896-902.
10. Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610-615.