User login
COVID-19 Monoclonal Antibody Infusions: A Multidisciplinary Initiative to Operationalize EUA Novel Treatment Options
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
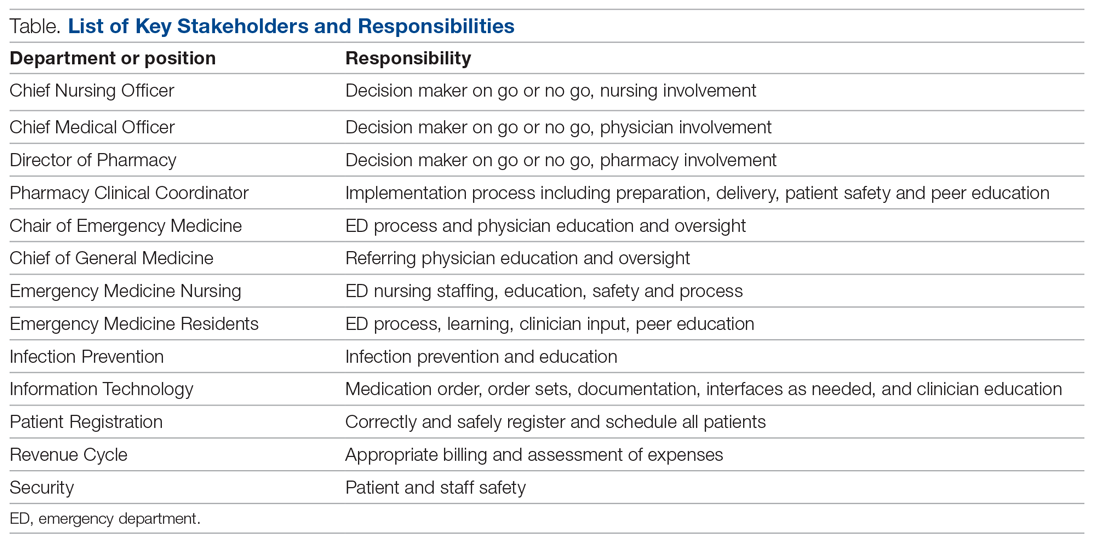
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
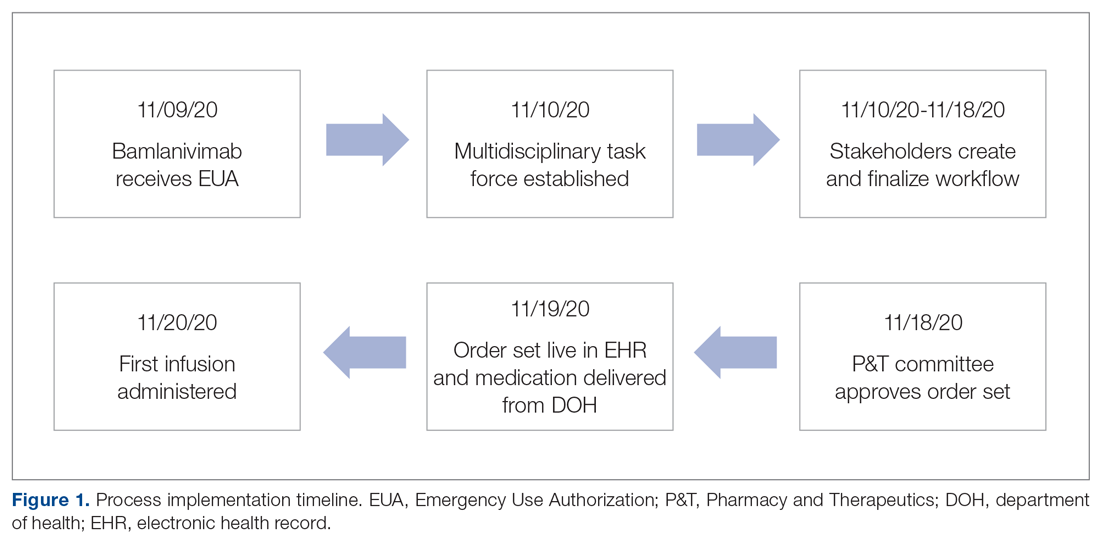
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
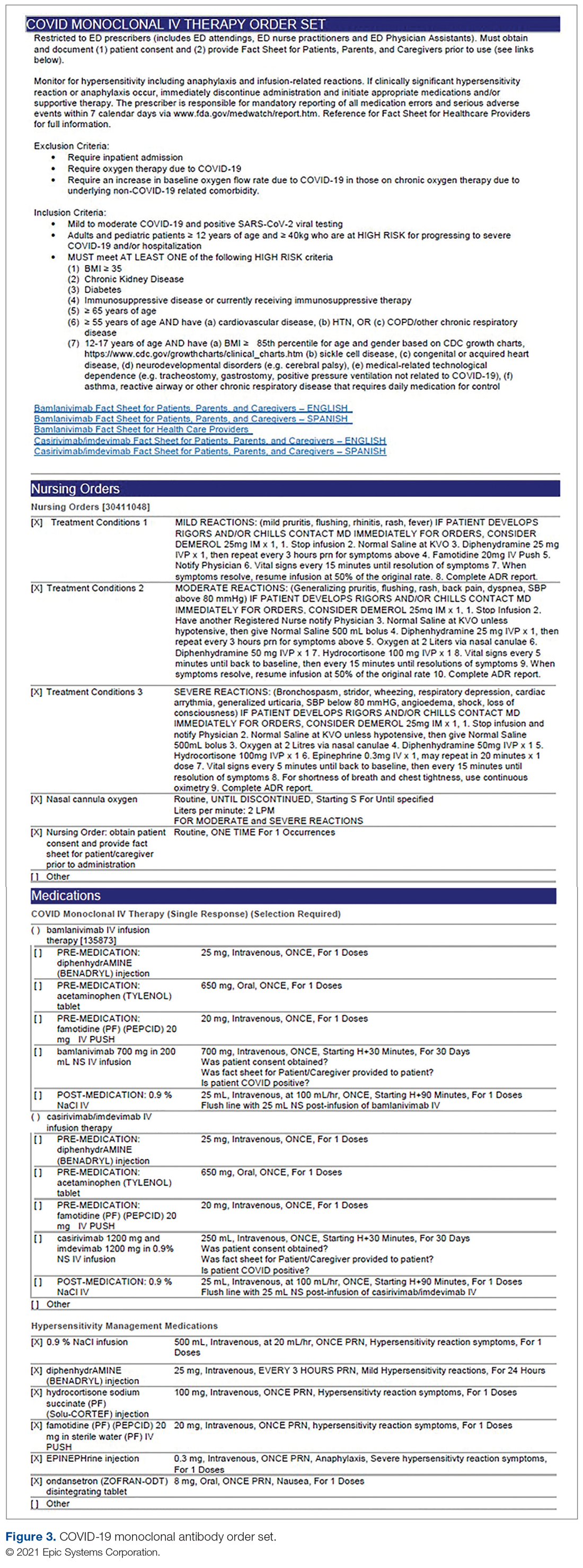
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
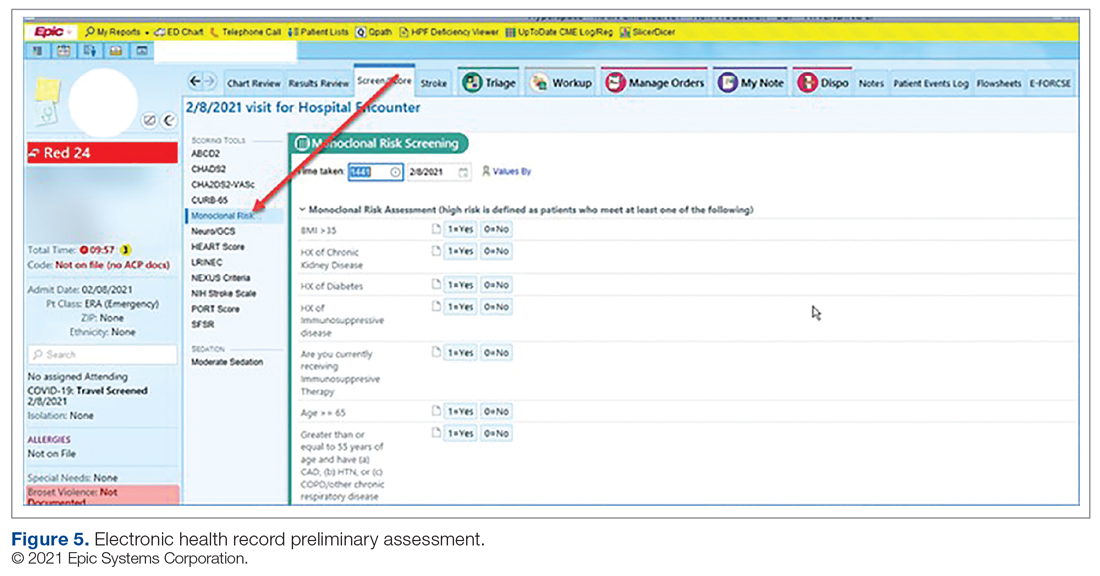
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
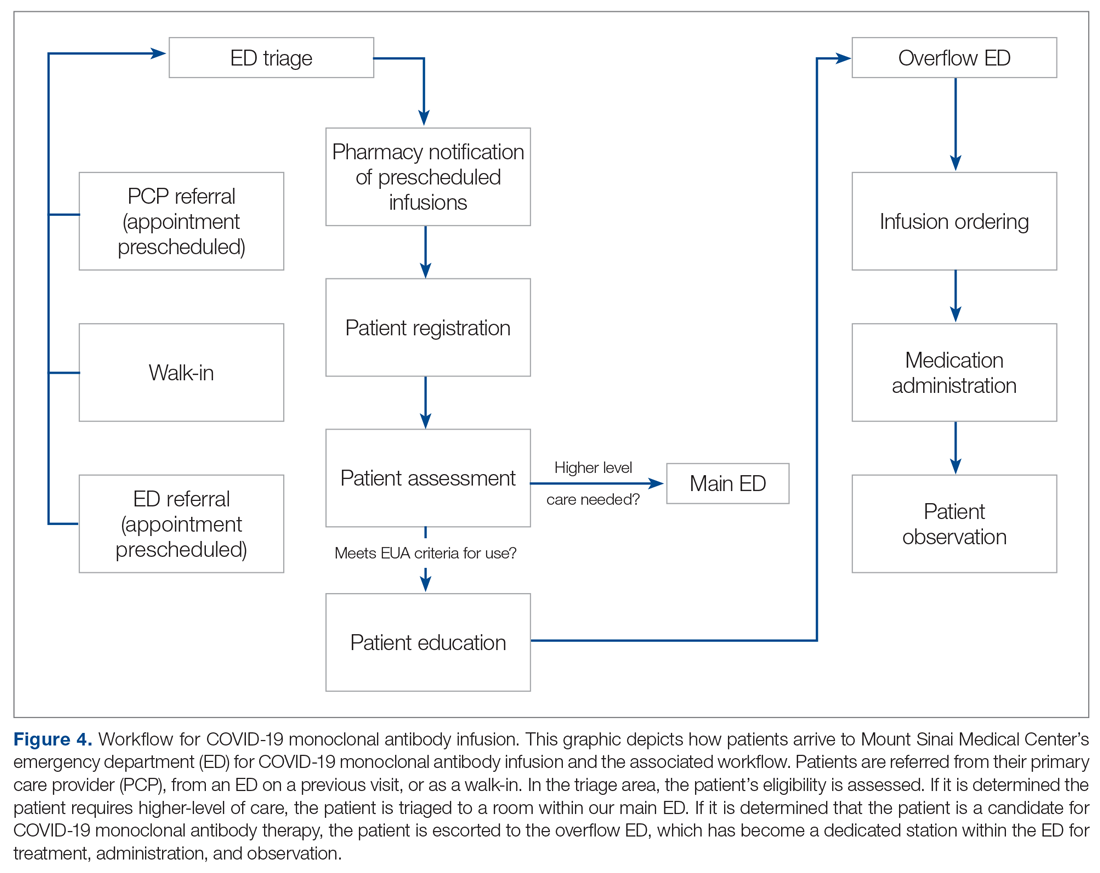
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; kathleen.jodoin@msmc.com.
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; kathleen.jodoin@msmc.com.
Financial disclosures: None.
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; kathleen.jodoin@msmc.com.
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
Review: Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.