User login
Population Management of Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
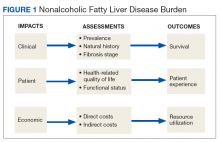
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
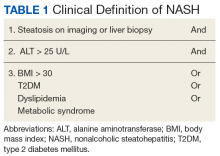
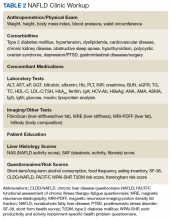
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
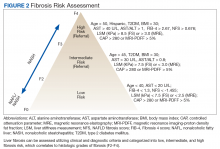
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
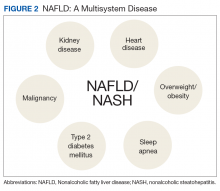
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
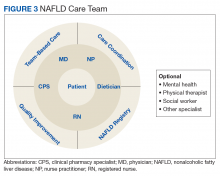
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
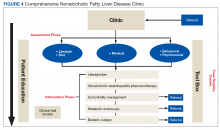
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both?
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.