User login
Promoting diversity through the AGA
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6
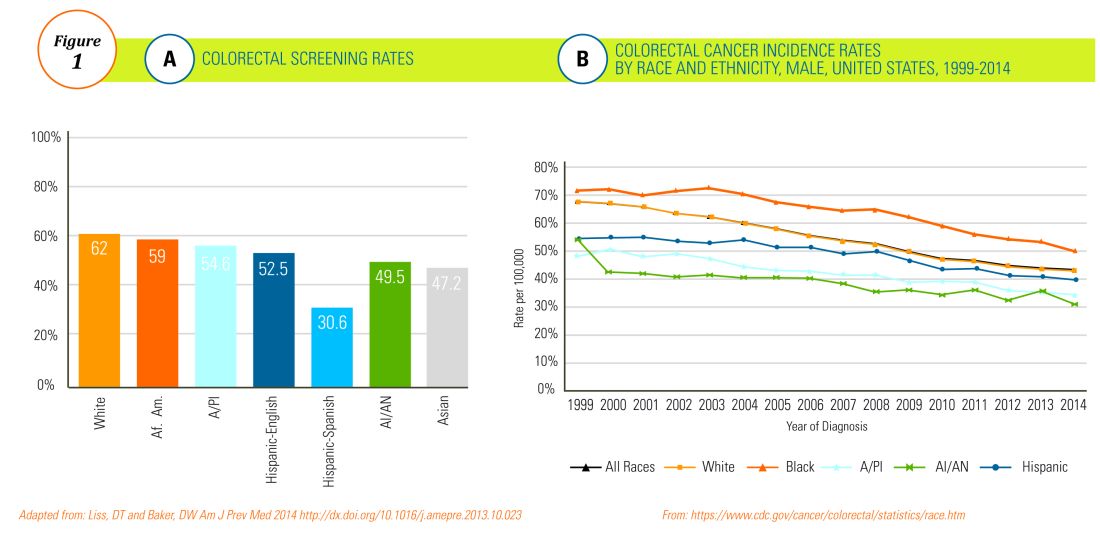
The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.
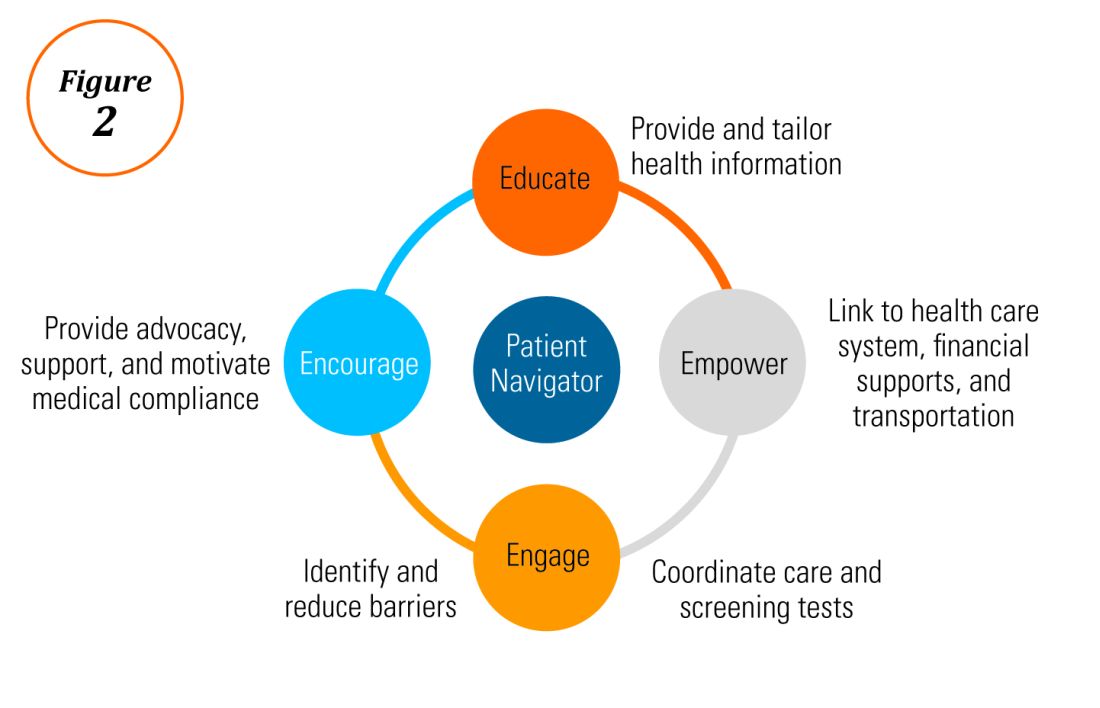
Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.

















