User login
Inflammatory Changes in Actinic Keratoses Associated With Afatinib Therapy
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
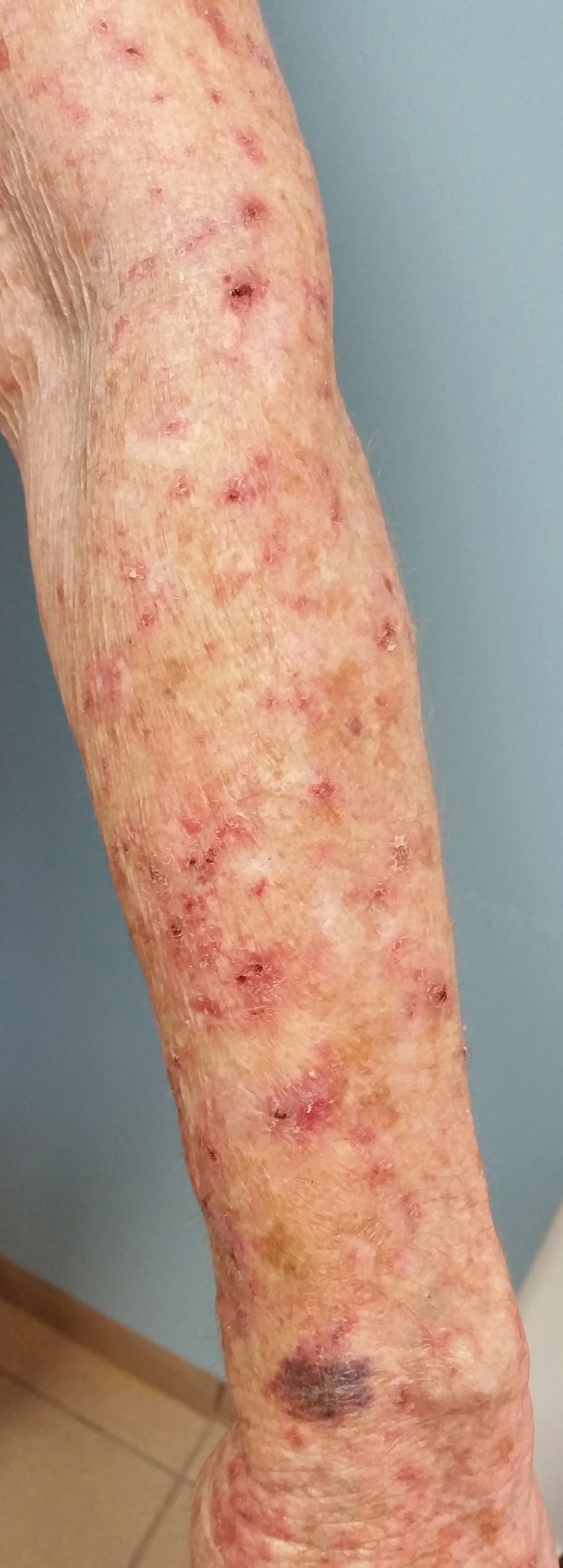
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
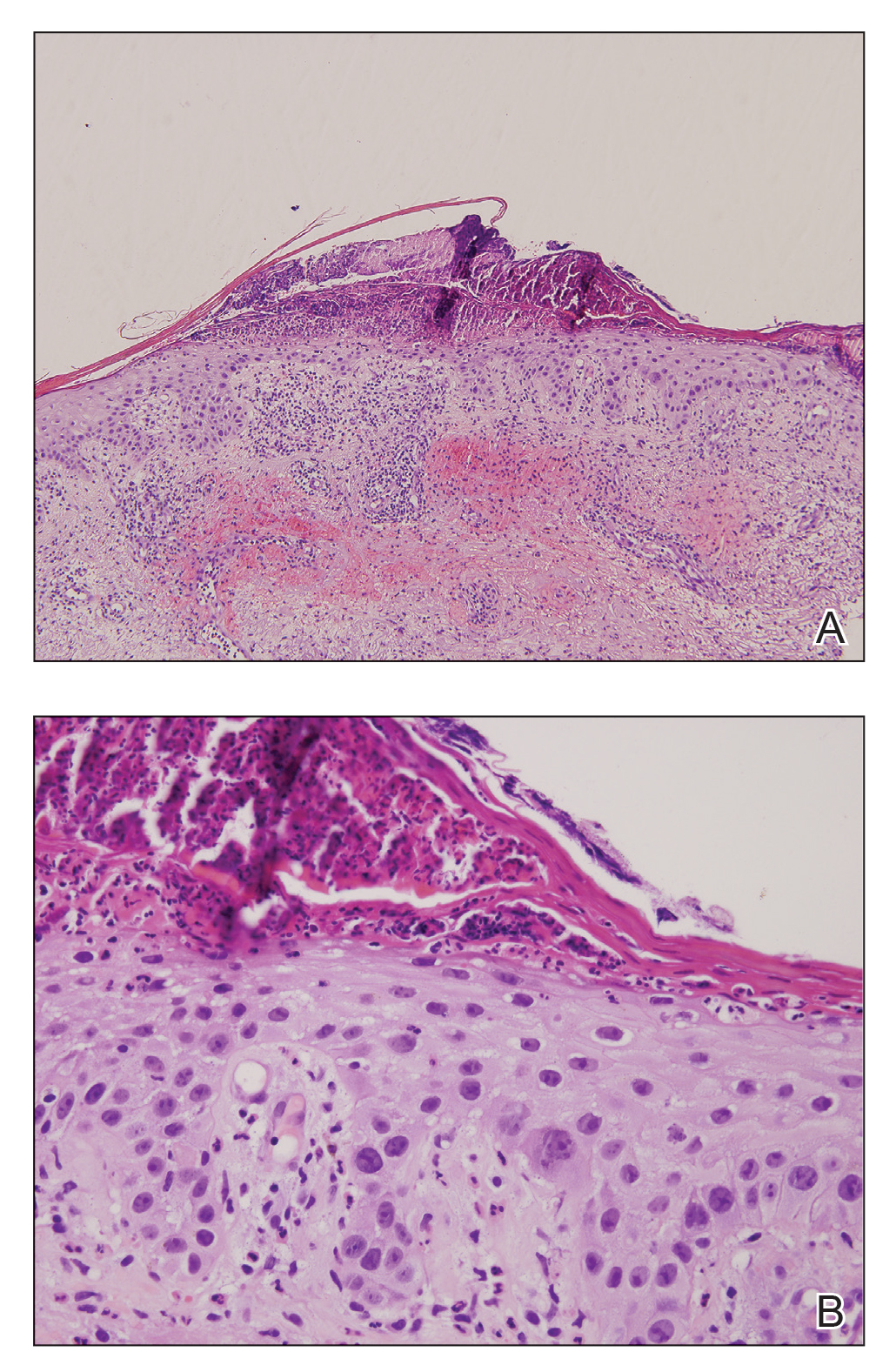
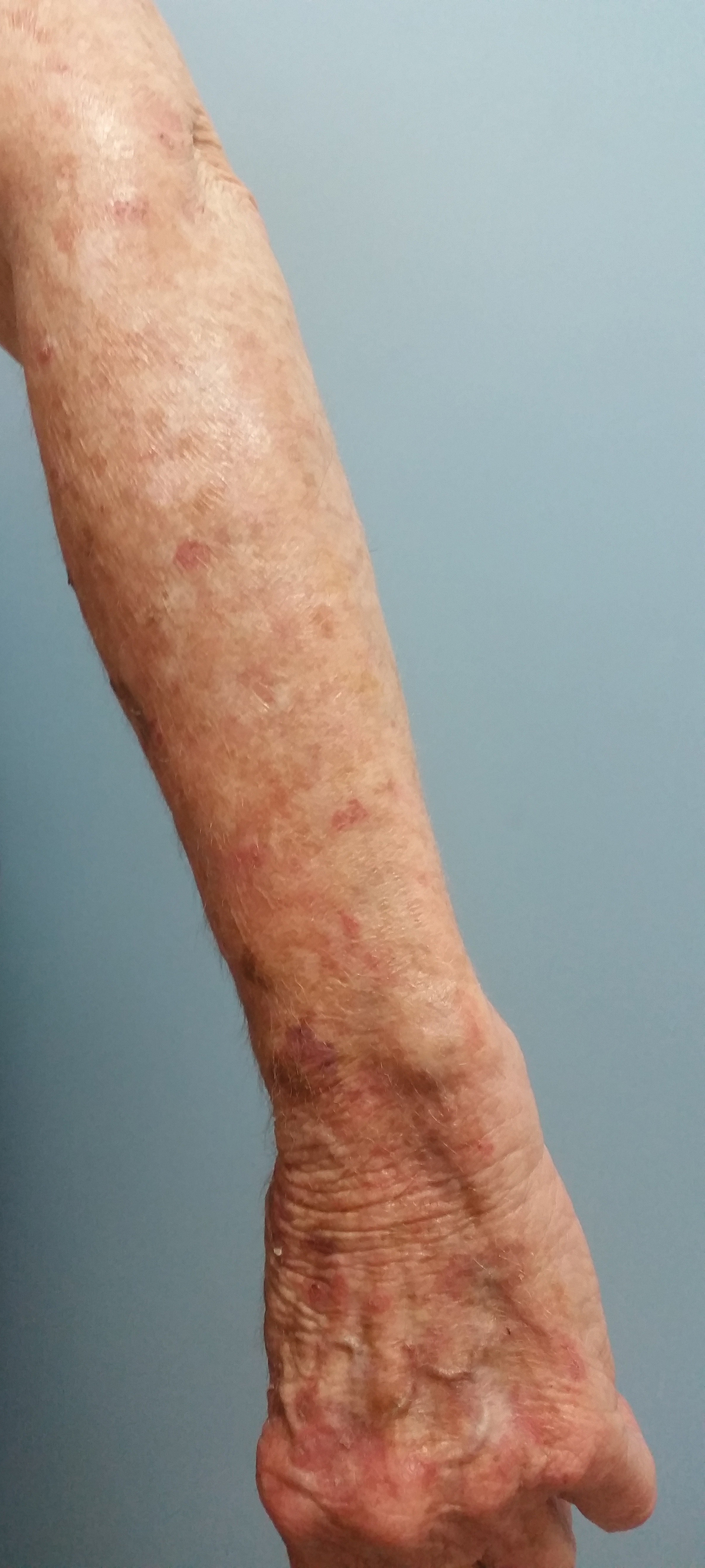
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
Practice Points
- One of the underreported adverse events of afatinibis is the induction of inflammatory changes in actinic keratoses (AKs).
- Our cases showed that inflammatory changes eventually led to shrinkage and resolution of the underlying AK.