User login
Considerations on the mode of delivery for pregnant women with hepatitis C infection
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
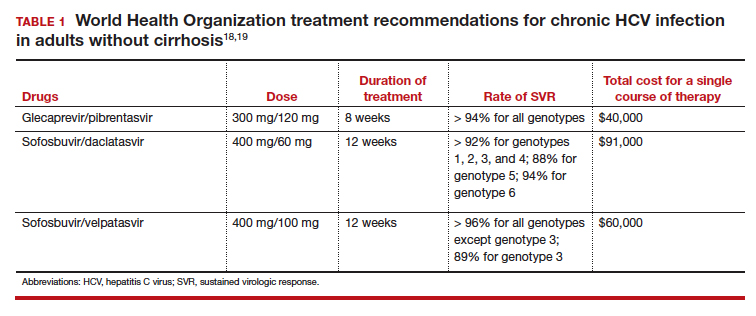
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11
Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
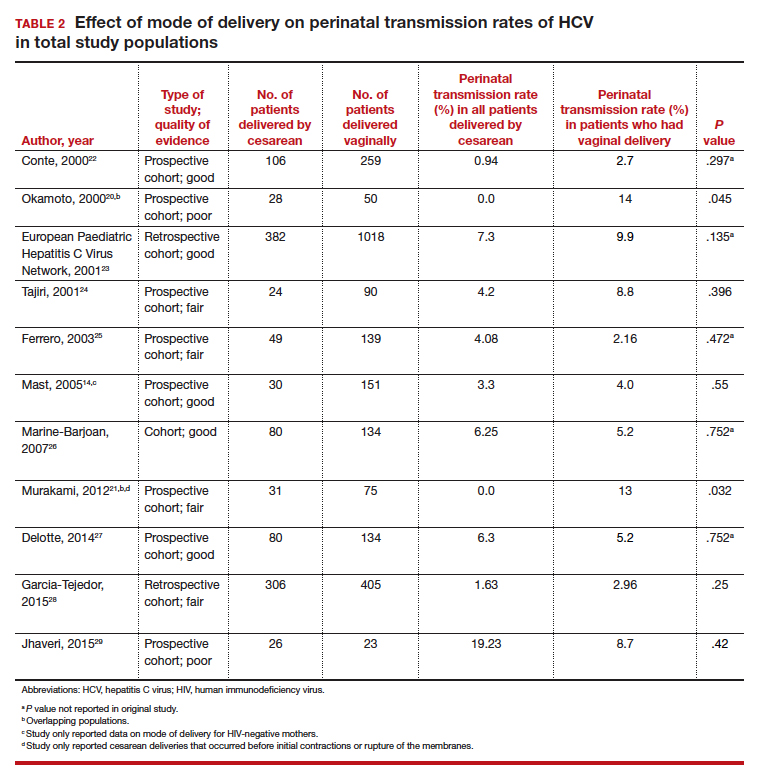
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
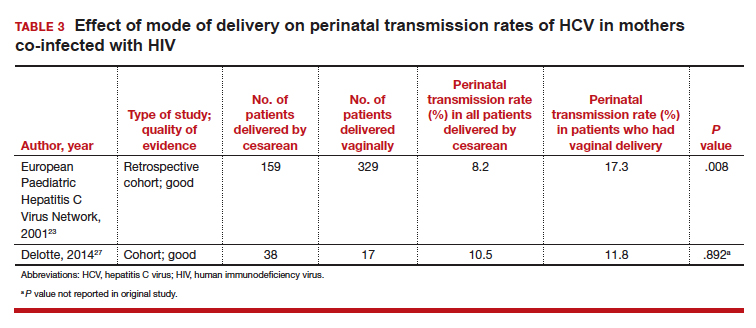
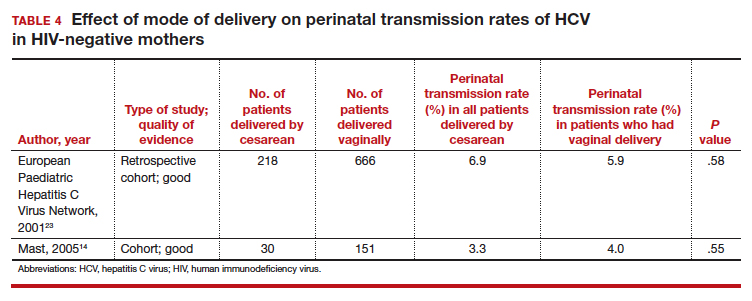
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
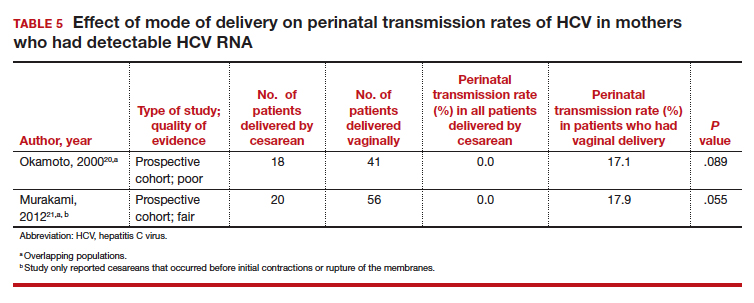
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
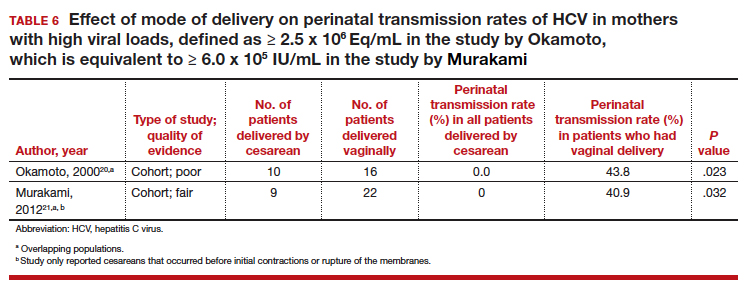
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21
Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.