User login
Safe Transitions and Congregate Living in the Age of COVID-19: A Retrospective Cohort Study
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
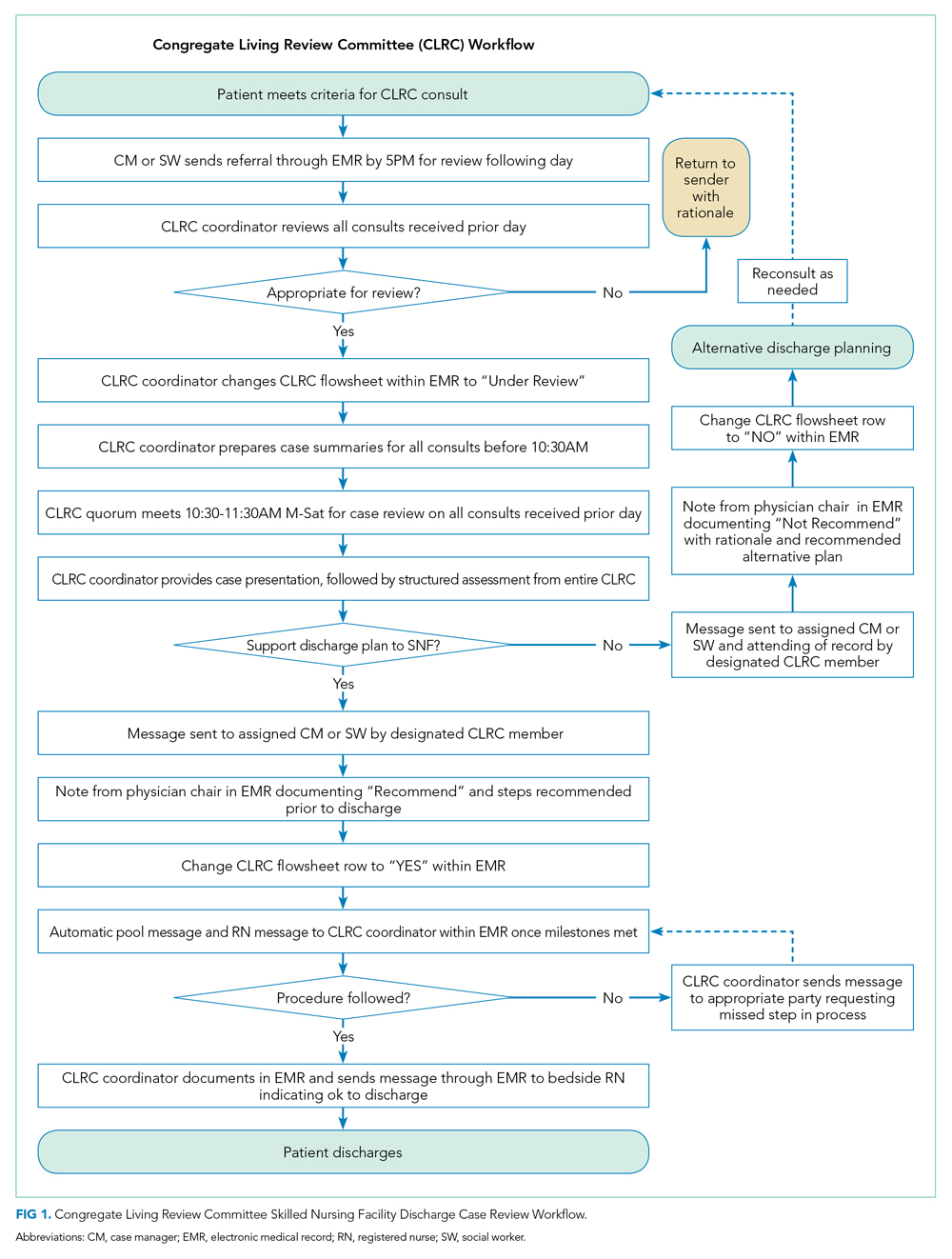
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
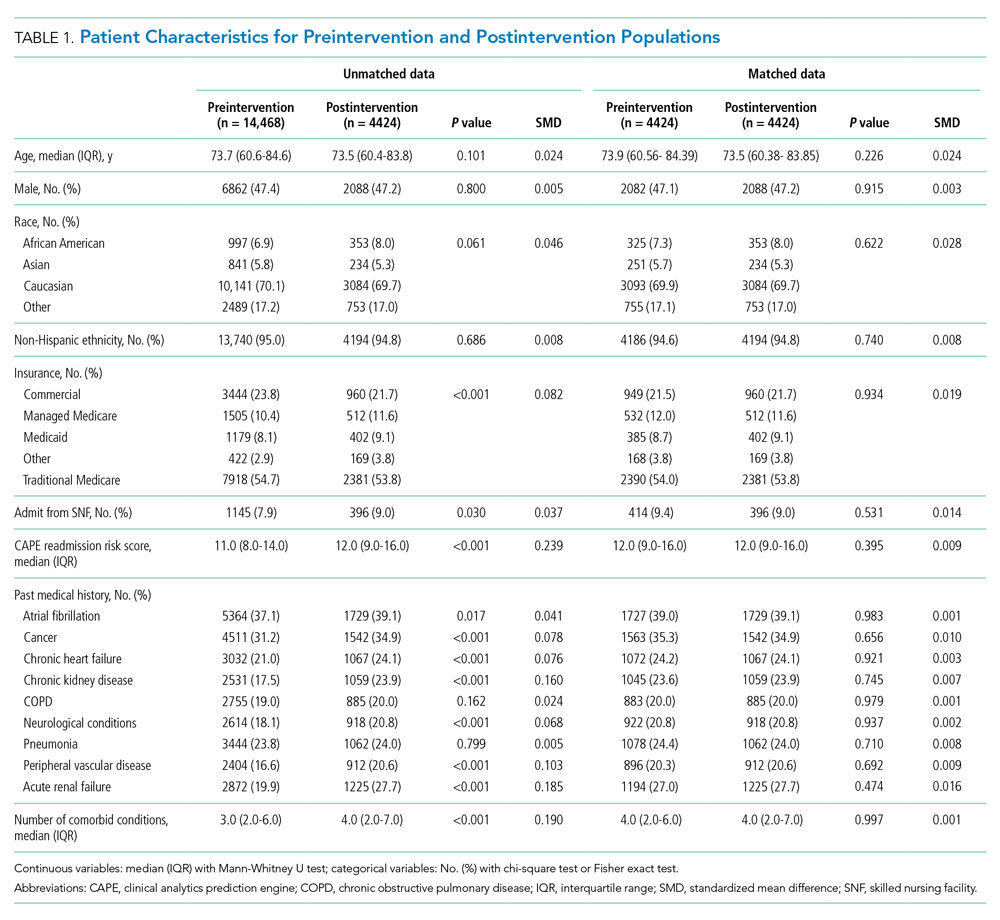
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
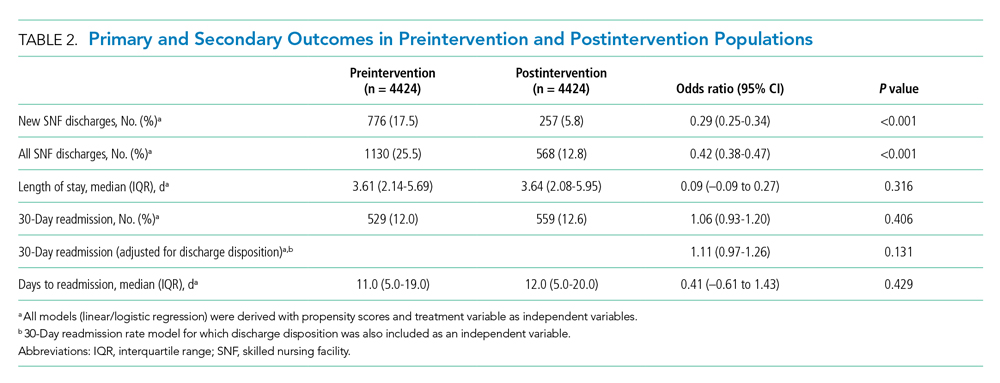
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
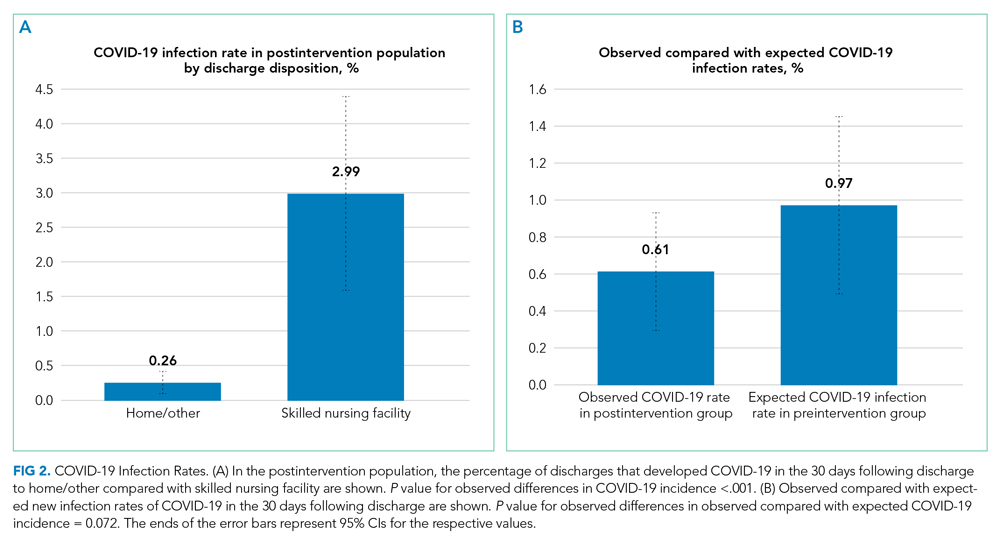
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
© 2021 Society of Hospital Medicine