User login
Halal nail polish
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
‘Clean’ and ‘natural’ beauty products
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
CBD in beauty products
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Windshield and UV exposure
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Piercing art
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
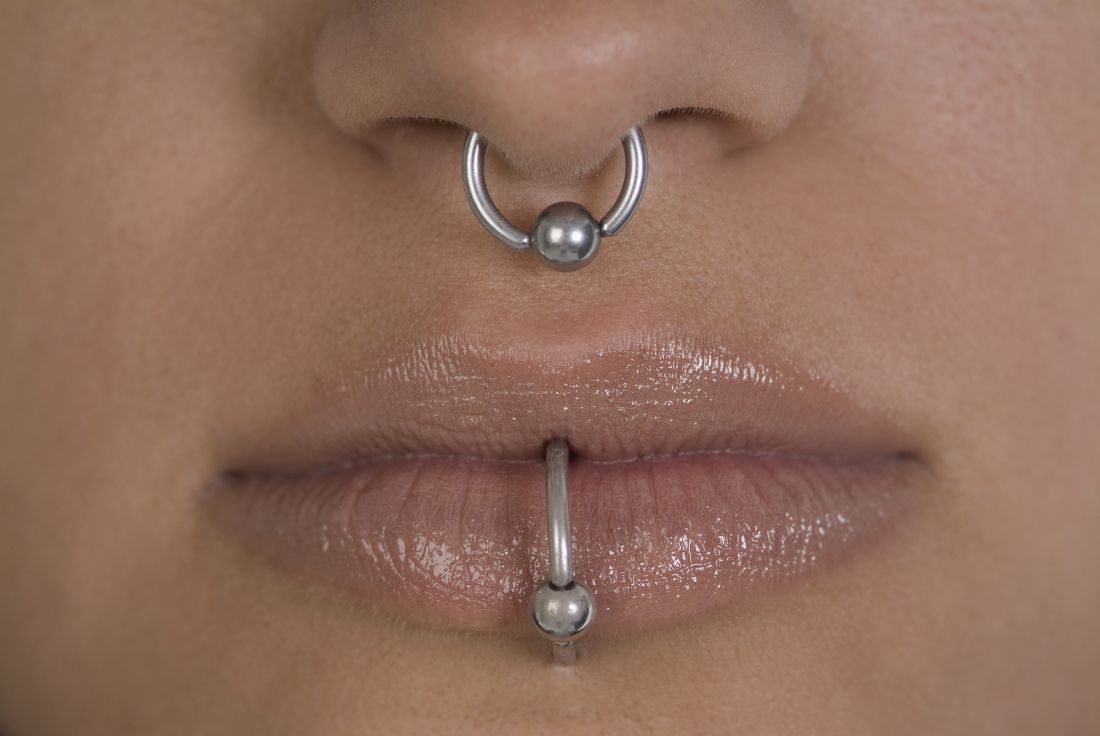
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Are nutritional supplements important in the treatment of female pattern hair loss?
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
The great sunscreen ingredient debate
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
Nitrous oxide in dermatology
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).
Wedding dermatology
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.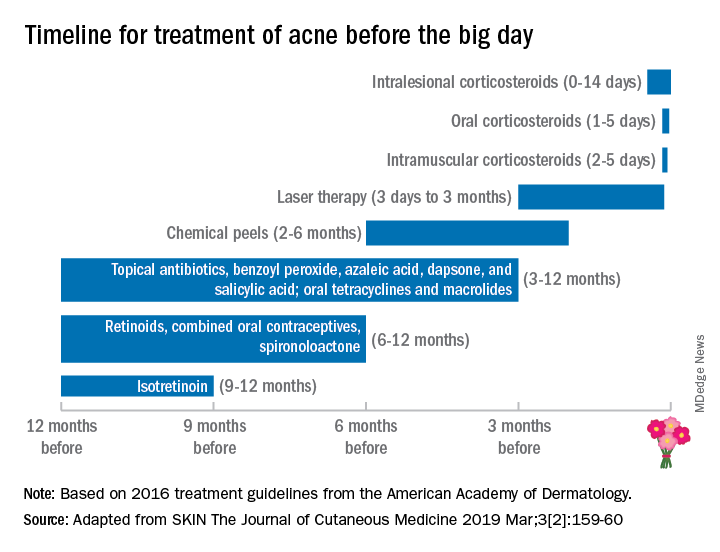
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Probiotic, prebiotic, and postbiotic skin care
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.