User login
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
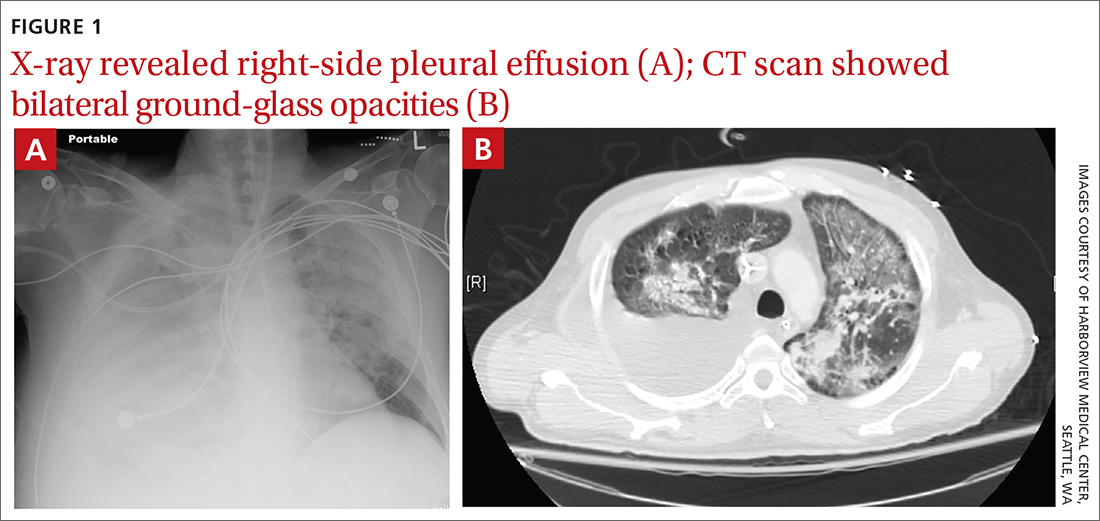
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
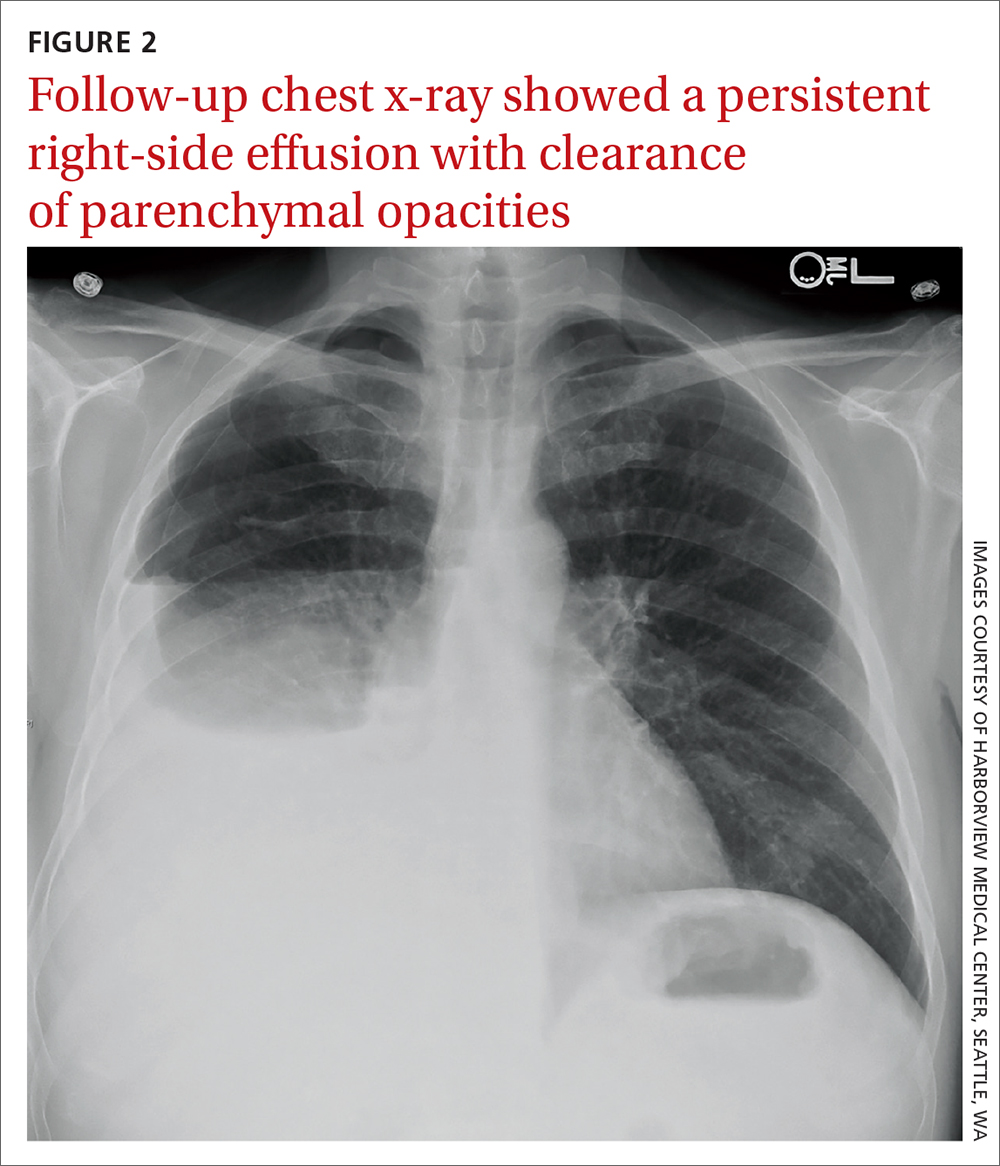
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042