User login
Things We Do for No Reason: Routine Echocardiography in Hemodynamically Stable Patients with Acute Pulmonary Embolism
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
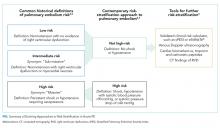
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
© 2019 Society of Hospital Medicine