User login
Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world's leading diabetes organizations
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
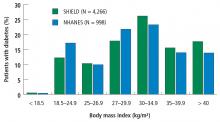
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
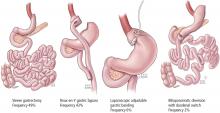
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
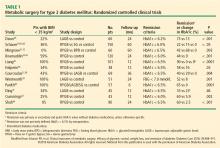
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
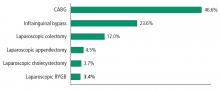
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
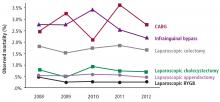
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
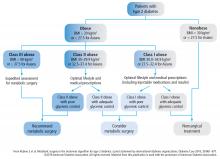
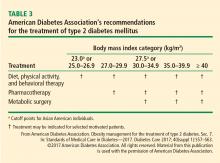
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
LIMITATIONS OF LIFESTYLE MANAGEMENT AND MEDICATIONS
First-line therapy with lifestyle management and second-line therapy with medications, including oral agents and insulin, are the mainstays of type 2 DM therapy. Although these approaches have reduced hyperglycemia and cardiovascular mortality, many patients have poor glycemic control and develop severe diabetes-related complications. A study using data from the National Health and Nutrition Examination Survey (N = 4,926) to evaluate success rates of lifestyle management plus drug therapy found that just 53% of patients with type 2 DM maintained a hemoglobin A1c (HbA1c) below 7%.6 Similarly, only 51% of those patients achieved a systolic and diastolic blood pressure less than 130/80 mm Hg, and only 56% achieved a low-density lipoprotein cholesterol level less than 100 mg/dL. Altogether, only 19% of the study cohort achieved all 3 therapy targets. Documented limitations of lifestyle counseling and drug therapy include behavior maladaptation, limitations in drug potency, nonadherence to medications, adverse effects, and economic deterrents.7
METABOLIC SURGERY FOR TYPE 2 DM
For patients with obesity and type 2 DM in whom lifestyle management and medications do not achieve desired treatment goals, bariatric surgery has emerged as the most effective treatment for attaining significant and durable weight loss. These gastrointestinal (GI) procedures, which reduce gastric volume with or without rerouting nutrient flow through the small intestine, were developed to yield long-term weight loss in patients with severe obesity. It is now known that they also cause dramatic improvement or remission of obesity-related comorbidities, especially type 2 DM. Research has shown that these effects are not only secondary to weight loss but also depend on neuroendocrine mechanisms secondary to changes in GI physiology. For these reasons, bariatric surgery is increasingly used with the primary intent to treat type 2 DM or metabolic disease, a practice referred to as metabolic surgery.
For more than 2 decades, indications for metabolic surgery reflected guidelines from a 1991 National Institutes of Health (NIH) consensus conference, which suggested considering surgery only in patients with a BMI of 40 kg/m2 or greater or a BMI of 35 kg/m2 or greater and significant obesity-related comorbidities.11 Guidelines published in 2013 expanded the recommendations to include adults with a BMI of at least 35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight.4 These recommendations were primarily designed to guide the use of surgery as a weight-loss intervention for severe obesity. However, guidelines published in 2016 support use of metabolic surgery as a specific treatment for type 2 DM.5
Potential mechanisms resolving type 2 DM: More than weight loss
Bariatric surgery has been shown to have profound glucoregulatory effects. These include rapid improvement in hyperglycemia and reduction in exogenous insulin requirements that occur early after surgery and before the patient has any significant weight loss.12,13 Additionally, experiments in rodents showed that changes to GI anatomy can directly influence glucose homeostasis, independently of weight loss and caloric restriction.14
Although the exact molecular mechanisms underlying the effects of metabolic surgery on diabetes are not fully understood, many factors appear to play a role, including changes in bile acid metabolism, GI tract nutrient sensing, glucose utilization, insulin resistance, and intestinal microbiomes.15 These changes, acting through peripheral or central pathways, or perhaps both, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced beta-cell function. A constellation of gut-derived neuroendocrine changes, rather than a single overarching mechanism, is the likely mediator of postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure.
METABOLIC SURGERY OUTCOMES
Weight loss
Long-term reduction of excess body fat is a major goal of metabolic and bariatric surgery. Weight loss is usually expressed as either the percent of weight loss or the percent of excess weight loss (ie, weight loss above ideal weight). A meta-analysis of mostly short-term weight-loss outcomes (ie, < 5 years) from more than 22,000 procedures found an overall mean excess weight loss of 47.5% for patients who underwent LAGB, 61.6% for RYGB, 68.2% for vertical-banded gastroplasty, and 70.1% for BPD-DS.16 Vertical-banded gastroplasty differs from LAGB in that both a band and staples are used to create a small stomach pouch. Excess weight loss for SG generally averages 50% to 55%, which is intermediate between LAGB and RYGB.17,18
The Swedish Obese Subjects study (N = 4,047), a prospective study of bariatric surgery vs nonsurgical weight management of severely obese patients (BMI > 34), is the largest weight-loss study with the longest follow-up.19 At 20 years, the mean weight loss was 26% for gastric bypass, 18% for vertical-banded gastroplasty, 13% for gastric banding, and 1% for controls. A 10-year study in 1,787 severely obese patients (BMI ≥ 35) who underwent RYGB had 21% more weight loss from their baseline weight than the nonsurgical match.20 At 4-year follow-up in 2,410 patients, there were significant variations in weight loss depending on the procedure: 27.5% for RYGB, 17.8% for SG, and 10.6% in LAGB. Between 2% and 31% regained weight back to baseline: 30.5% for LAGB, 14.6% for SG, and 2.5% for RYGB.20 In contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5%, even with intensive lifestyle intervention.21
Diabetes remission, cardiovascular risk factors, glycemic control
A meta-analysis of 19 mostly observational studies (N = 4,070 patients) reported an overall type 2 DM remission rate of 78% after bariatric surgery with 1 to 3 years of follow-up.22 Resolution or remission was typically defined as becoming “nondiabetic” with normal HbA1c without medications. In the Swedish Obese Subjects study, the remission rate was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical controls (P < .001).23 Bariatric surgery was also markedly more effective than nonsurgical treatment in preventing type 2 DM, with a relative risk reduction of 78%.
A systematic review published in 2012 evaluated long-term cardiovascular risk reduction after bariatric surgery in 73 studies and 19,543 patients.24 At a mean follow-up of 57.8 months, the average excess weight loss for all procedures was 54% and rates of remission or improvement were 63% for hypertension, 73% for type 2 DM, and 65% for hyperlipidemia. Results from 12 cohort-matched, nonrandomized studies comparing bariatric surgery vs nonsurgical controls suggest that improvements in surrogate disease markers such as HbA1c, blood pressure, lipids, and body weight after surgery translate to reduced macrovascular and microvascular events and death.25 One of these studies involving male veterans who were mostly at high cardiovascular risk reported a 42% reduction in mortality at 10 years compared with medical therapy.26
In the Swedish Obese Subjects study, the mortality rate from cardiovascular disease in the bariatric surgical group was lower than for control patients (adjusted hazard ratio, 0.47; P = .002) despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort.19 For patients with type 2 DM in this study, surgery was associated with a 50% reduction in microvascular complications.27 After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years for control patients and 20.6 per 1,000 person-years in the surgery group (hazard ratio, 0.44; P < .001).
These observational, nonrandomized study data suggest that in patients with type 2 DM, bariatric surgery is significantly better than medical management alone in improving glycemic control, reducing cardiovascular risk factors, and lowering long-term morbidity and mortality associated with type 2 DM.
METABOLIC SURGERY: CLINICAL TRIALS
Collectively, these RCTs showed that surgery was significantly superior to medical treatment in reaching the designated glycemic target (P < .05 for all). The one exception showed that diabetes remission for LAGB vs medical treatment was 33% and 23%, respectively.41 This result might be due to patients in this study having advanced type 2 DM (HbA1c 8.2% ± 1.2%, with 40% on insulin), and they likely had reduced beta-cell function. Overall, surgery decreased HbA1c by 2% to 3.5%, whereas medical treatment lowered it by only 1% to 1.5%. Most of these studies also showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, lipids, and quality of life. Results were mixed in terms of improvements in systolic and diastolic blood pressure or low-density lipoproteins after surgery vs medical treatment, but many studies did show a corresponding reduction in medication usage.
Durability of the effects of surgery was demonstrated in a 5-year study that showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD in severely obese patients (BMI ≥ 35) vs medical therapy.32 Similarly, Schauer et al43 showed that RYGB and SG were more effective than intensive medical therapy in improving or, in some cases, resolving hyperglycemia for 5 years. In the RCTs, patients who preoperatively had shorter duration of diabetes, lower HbA1c levels, no insulin requirement, and more postoperative weight loss were more likely to achieve diabetes remission.
Although previous guidelines and payer coverage policies had limited metabolic surgery to severely obese patients (BMI ≥ 35 kg/m2), nearly all RCTs showed that the surgical procedures, especially RYGB and SG, were equally effective in patients with BMI 30 to 35 kg/m2. This is particularly important given that most patients with type 2 DM have a BMI less than 35 kg/m2. The effect of surgery in these patients with mild obesity is also durable out to at least 5 years.43
No RCT was sufficiently powered to detect differences in macrovascular or microvascular complications or death, especially at the relatively short follow-up, and no such differences have been detected thus far. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial43 showed that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 5 years compared with intensive medical management.
METABOLIC SURGERY: ADVERSE EVENTS
Surgical complications
Nutritional deficiencies
Postoperative nutritional deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures. They are more common after RYGB and BPD-DS and less common after SG and LAGB. In addition, there is a high prevalence of nutritional deficiencies (35%–80%) in patients seeking bariatric surgery; thus, poor preoperative nutrition may be a factor in the development of postoperative deficiencies. Common preoperative nutrient deficiencies are vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%).51 Recommendations are to assess for these deficiencies and correct any identified before surgery.
Mild anemia after bariatric procedures is common, occurring in 15% to 20% of cases, and it is believed to result from reduced absorption of iron and B12, as well from pre-existing iron deficiency anemia in premenopausal patients.52 Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can occur after bariatric procedures, especially after BPD-DS.53 Nutrient deficiencies can be prevented or corrected with appropriate vitamin, iron, and calcium supplementation.54
Bone mineral density may decrease after bariatric surgery (14% in the proximal femur).55 Reduced mechanical loading after weight loss, reduced consumption and malabsorption of micronutrients (calcium, vitamin D), and neurohormonal alterations are potential underlying mechanisms of bone mineral density reduction after bariatric surgery. Rates of bone fracture and osteoporosis are not well delineated, raising questions about whether bone loss after bariatric surgery is clinically relevant or a functional adaptation to skeletal unloading. However, the extreme malabsorptive procedures of BPD-DS have been associated with severe calcium and vitamin D deficiencies, leading to decreased bone mineral density and osteoporosis.
Protein malnutrition also can occur after these extreme malabsorptive procedures. Patients require postoperative oral protein supplementation (80–100 g/day) and lifelong monitoring for nutritional complications after these procedures.56
Additional complications
Other late complications of bariatric surgery that are less clear in incidence and cause include kidney stones, alcohol abuse, depression, and suicide. One study of patients after RYGB (N = 4,690) reported a significantly higher prevalence of kidney stones than in obese controls: 7.5% vs 4.6%, respectively.57 Proposed causes of kidney stone formation following bariatric surgery include hyperoxaluria, hypocitraturia, and elevated urine acidity.58
The prevalence of alcohol-use disorder after bariatric surgery ranges from 7.6% to 11.8% and appears to be higher in patients with a history of alcohol use.59 Paradoxically, while bariatric surgery has been shown to significantly decrease depression,60 some studies suggest that a slight increase in the risk of suicide may occur,61 while others do not.62 A recent review concluded that accurate rates of suicide after bariatric surgery are not known, but practitioners should be aware of this concern and appropriately screen and counsel their patients.63
Although the 12 RCTs reported in Table 1 were not powered to detect differences in treatment-related complications, the overall rates of complications were consistent with those in observational studies.9 The most common surgical complications were anemia (15%), need for reoperation (8%), and GI (5%–10%). The 30-day surgical mortality rate was 0.2% (1 death) among the 465 surgical patients. Complications were not limited to the surgical patients. In the medical-treatment control group of the STAMPEDE trial,30 anemia (16%) and weight gain (16%) were common. Investigators reported challenges with medication compliance, including adverse effects leading to discontinuation of medications. Mild hypoglycemia was common, with no significant differences between the surgical and medical treatment groups.
METABOLIC SURGERY: COST EFFECTIVENESS
The cost of bariatric procedures varies considerably but, in general, ranges from $20,000 to $30,000, similar to the cost of cholecystectomy, hysterectomy, and colectomy. Retrospective analyses and modeling studies indicate that metabolic surgery is cost-effective and may present a cost savings in patients with type 2 DM, with a break-even time between 5 and 10 years.64,65 The cost savings, largely based on assumptions of long-term effectiveness and safety, result from reductions in medication use, outpatient care costs, and long-term complications of type 2 DM.
WHO SHOULD HAVE METABOLIC SURGERY?
Until recently, there was no clear national or international consensus on the role of metabolic surgery in treating type 2 DM. In 2015, the 2nd Diabetes Surgery Summit (DSS-II) Consensus Conference published guidelines that were endorsed by more than 50 diabetes and medical organizations.5 The recommendations cover many clinically relevant issues, including patient selection, preoperative evaluation, choice of procedure, and postoperative follow-up. The consensus conference delegates concluded that there is sufficient evidence demonstrating that metabolic surgery achieves excellent glycemic control and reduces cardiovascular risk factors.
The treatment algorithm from DSS-II incorporates appropriate use of all 3 treatment modalities: lifestyle intervention, drug therapy, and surgery (Figure 5).5 The 2017 Standards of Care for Diabetes from the American Diabetes Association include those key indications in the recommendations for metabolic surgery (Table 3).2
SUMMARY
The safety of metabolic surgery has significantly improved with the advent of laparoscopic surgery and recent national quality improvement initiatives that have made gastric bypass and SG as safe as cholecystectomy and appendectomy. Although observational studies suggest that metabolic surgery is associated with a reduction in cardiovascular and diabetes complications and mortality, these observations have not been confirmed in long-term RCTs.
Based on the published evidence, metabolic surgery is now endorsed as a standard treatment option, which provides patients and practitioners with a powerful tool to help combat the life-impairing effects of type 2 DM.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
- Bays HE, Chapman RH, Grandy S; for the SHIELD Investigators Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract May 2007; 61:737–747.
- Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes—2017. Diabetes Care 2017; 40(suppl 1):S1–S135.
- Fox CS, Golden SH, Anderson C, et al; American Heart Association; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015; 132:691–718.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016; 39:861–877.
- Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36:2271–2279.
- Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014; 35:3267–3276.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832.
- Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39:902–911.
- Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015; 11(suppl):S1–S34 [Abstract A111].
- Consensus Development Conference Panel. NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991; 115:956–961.
- Pories WJ, MacDonald KG Jr, Flickinger EG, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215:633–642.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239:1–11.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016; 39:893–901.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5:469–475.
- Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012; 256:262–265.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307:56–65.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016; 151:1046–1055.
- Wing RR, Bolin P, Brancati FL, et al; for the Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369:145–154.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256.
- Sjöström L, Lindroos AK, Peltonen M, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693.
- Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98:1763–1777.
- Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127:945–959.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311:2297–2304.
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299:316–323.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomized controlled trial. Lancet 2015; 386:964–973.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomized, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422.
- Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2:545–552.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014; 260:617–622.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100:2546–2556.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016; 59:945–953.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Metabolic surgery vs. intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med 2017; 376:641–651.
- Shah SS, Todkar J, Phadake U, et al. Gastric bypass vs. medical/lifestyle care for type 2 diabetes in South Asians with BMI 25-40 kg/m2: the COSMID randomized trial [261-OR]. Presented at the American Diabetes Association’s 76th Scientific Session; June 10–14, 2016; New Orleans, LA.
- Flum DR, Belle SH, King WC, et al; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009; 361:445–454.
- Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015; 17:198–201.
- Thodiyil PA, Yenumula P, Rogula T, et al. Selective non operative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008; 248:782–792.
- Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007; 21:1914–1918.
- Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19:1702–1706.
- Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011; 146:802–807.
- Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006; 16:603–606.
- Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008; 83:403–409.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26:1031–1037.
- Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008; 18:1062–1066.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014; 16:1204–1213.
- Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013; 29:684–693.
- Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181:2573–2577.
- Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84:1–8.
- Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016; 76:16–29.
- Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015; 11:637–342.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013; 21:665–672.
- Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016; 96:669–679.
- Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016; 39:954–963.
KEY POINTS
- Randomized clinical trials have shown that metabolic surgery is statistically superior to medical treatment in achieving targeted glycemic levels along with improvements in weight loss, remission of metabolic syndrome, reduction in medications, and improvements in lipid levels.
- The safety of metabolic and bariatric surgery has significantly improved with the advent of laparoscopic surgery, resulting in complication profiles similar to those of cholecystectomy and appendectomy.
- Metabolic surgery is now recommended as standard treatment option for type 2 diabetes in patients with body mass index levels as low as 30 kg/m2.
Inside the operating room—balancing the risks and benefts of new surgical procedures
How should we introduce and evaluate new procedures?
By Joel D. Cooper, MD
Time magazine published an article in 1995 titled “Are Surgeons Too Creative?” that examined the question of whether operations should be regulated the way that medications are.1 The piece featured two patients. One, a patient with emphysema who underwent lung volume reduction surgery at our institution during the early days of this procedure, had a good outcome. The other was a neurosurgical patient who had a bad outcome.
The public is somewhat sympathetic to this article’s premise, which can be viewed as a call to require a similar level of evidence for surgical procedures as for new drugs. This sympathy arises from the expense of new technologies, pressure from payors to control costs and increase profits, hospital budget restraints, and the reality of increasingly well-informed patients.
Yet there are distinct differences between drugs and surgery. A new drug does not change over time. A new drug is associated with a variable biologic response whose assessment often requires large numbers of patients and considerable follow-up. And a new drug may manifest unforeseen late side effects and toxicities far removed from the time of initial use. In contrast, none of these characteristics applies to surgical procedures. A surgical intervention changes over time as the technique and experience evolve and as refinements are made in patient selection and in pre- and postoperative management. With this evolution comes a change in risk over time. Patient selection for surgery is as much an art as a science; each patient requires assessment of both the potential benefits and risks of the procedure, which argues against offering an operation by prescription. Moreover, with surgery, the facilities and the operator’s skill and experience levels vary from one center to another.
INTRODUCTION OF NEW PROCEDURES: COVERAGE VS VALIDATION
Introduction of a new surgical procedure depends on the nature of the procedure and the other interventions that may be available for the condition. In assessing how new procedures should be introduced, I believe we need to distinguish between coverage and validation. Coverage—ie, payment for the procedure—is an economic issue, whereas validation involves an ethical and scientific evaluation of the role of the procedure.
Coverage by an insurer should have at least theoretical justification and presumption of benefit. For instance, the rationale behind a heart transplant for a patient with a failing heart is obvious. Coverage generally requires preliminary evidence of efficacy, possibly in an animal model, although no animal models may exist for some conditions. Most important, a different standard for providing initial coverage should be applied if no alternative therapy exists for a condition that is severe, debilitating, and potentially life-threatening; if a new procedure treats a condition for which a standard therapy already exists, the standard for coverage must be higher. Finally, coverage in all cases should require ongoing reassessment of the procedure.
In contrast, validation is a scientific analysis of results over time, including long-term results, and can be accomplished by well-controlled case series, particularly if the magnitude of the benefit is both frequent and significant and especially if no alternative therapy exists. Randomized clinical trials are the gold standard for appropriate interventions but are not always applicable.
A 1996 study by Majeed et al2 provides a good example of validation-oriented surgical research. In this blinded trial, 200 patients scheduled for cholecystectomy were randomized to either laparoscopic or open (small-incision) procedures. The study found no differences between the groups in terms of hospital stay or postprocedure pain or recovery. In an accompanying commentary,3Lancet editor Richard Horton praised the design and conduct of the study, noting that it was very much the exception in surgical research, which he argued was preoccupied with case series. Horton offered the following speculation about this preoccupation:
Perhaps many surgeons do not see randomised trials as feasible strategy to resolve questions about surgical management. Cynics might even claim that the personal attributes that go to make a successful surgeon differ from those needed for collaborative multicentre research.3
IS THE ‘SURGICAL SCIENTIST’ AN OXYMORON?
Barnaby Reeves, writing in The Lancet 3 years later, offered a more diplomatic take on the difficulty of evaluating surgical procedures:
What makes a surgical technique new is not always easy to define because surgical procedures generally evolve in small steps, which makes it difficult to decide when a procedure has changed sufficiently to justify formal evaluation.4
Reeves went on to argue that doing an evaluation too early may preclude acceptance, since the technique may not have evolved sufficiently and surgeons may not have mastered it; conversely, doing an evaluation too late may make the evaluation moot, since the technique may have already become established and withholding it may be deemed unethical. Additionally, he noted that the quality of surgical evaluation is complicated by the possibility that some surgeons have better mastery of—and therefore better outcomes with—one procedure while other surgeons have better mastery and outcomes with an alternative procedure.4
These concerns were well captured by the late Dr. Judah Folkman, whom I once heard say, “When a basic scientist is informed that another investigator cannot reproduce his work, it has a chilling effect; for the surgeon, however, it is a source of pride.”
RANDOMIZED TRIALS VS CASE SERIES: A TIME AND PLACE FOR EACH
Even as we recognize these challenges specific to surgical evaluation, we are still left with the task of determining when a randomized controlled trial is appropriate and when a case-control series may suffice.
There are three broad sets of circumstances in which a randomized trial is essential:
- For preventive procedures, ie, when the operation is done to reduce the potential for a future adverse event. An example would be evaluating carotid endarterectomy to reduce the potential for stroke in asymptomatic patients with 60% or greater stenosis. Only a randomized trial could have shown a difference in favor of endarterectomy over aspirin plus best medical therapy.
- To compare a procedure with alternative medical or surgical interventions. I would argue that laparoscopic surgery should have been introduced with randomized trials, as it begs one to suspend judgment and accept that small incisions are invariably and de facto better than a large incision.
- For trials in oncology, where the outcome depends on long-term results, such as survival or time to recurrence. Examples would include comparisons of surgery alone versus surgery plus chemotherapy for prevention of cancer recurrence.
Similarly, there are several scenarios in which a case-control series is appropriate and adequate:
- When no alternative therapy exists. Falling into this category, in my view, are lung transplant, which we introduced successfully at the University of Toronto in 1983, and lung volume reduction surgery, which we introduced in 1993.
- When the natural history of the condition is well documented and the impact of the intervention is obvious.
- When the magnitude of the procedure’s effect is measurable, significant, and expected.
RANDOMIZED TRIALS IN SURGERY
Advantages of randomized trials
Randomized clinical trials confer a number of advantages. They eliminate bias. They ensure a balance between treatment groups in terms of known or unknown prognostic factors. And, importantly, they have a major impact on payors.
A tale of two Medicare payment decisions
The impact of clinical trials on payors is exemplified by the contrasting stories of two procedures: transmyocardial laser revascularization and lung volume reduction surgery.
Transmyocardial laser revascularization (TMR) involves the creation of channels in the myocardium with a laser to relieve angina. Although TMR is a dubious intervention with no physiologic rationale (similar to internal mammary artery ligation for angina5) and no proven improvement in life expectancy (only a reduction in pain), it was approved for reimbursement by Medicare because it was investigated in a randomized trial.6 However, the “randomized trial” was not truly a randomized investigation because the control patients received only medical therapy and did not go to the operating room to receive a sham operation.6 Despite this flaw, the perceived authority of the trial was sufficient to influence Medicare.
In contrast, Medicare refused to pay for lung volume reduction surgery until it was subjected to a randomized trial, despite the fact that the procedure had produced tremendous benefit in hundreds of patients at multiple centers who otherwise could not have achieved such benefit. Only after $50 million was spent on a randomized controlled trial, the National Emphysema Treatment Trial (NETT),7–9 did Medicare agree to pay for lung volume reduction surgery. The trial showed that over 5 years, the procedure was associated with significant improvements in life expectancy, exercise tolerance, and quality of life, but the study took 8 years to conduct and by then it was a bit too late, as detailed in the following section.
NETT: A case study in how a trial can be counterproductive
Lung volume reduction surgery is an operation based on the recognition that the crippling effects of emphysema are hyperinflation of the chest, flattening of the diaphragm, and inability to move air in and out of the chest. The notion that the chest can be reconfigured in the patient with emphysema by removing the distending overinflated emphysema led us to develop the volume reduction operation.
The NETT was initiated by Medicare, and the protocol denied compassionate crossover of patients.7 In an attempt to establish clinical equipoise, surgeons who participated were not allowed to perform any volume reduction operations on non-Medicare patients or on Medicare patients not enrolled in the trial. After 2 years of slow patient enrollment, the clinical trial committee, in an effort to increase enrollment, eliminated the original entrance criteria specifying certain degrees of hyperinflation and diffusing capacity. An excess of mortality was discovered 2 years later in a subgroup randomized to volume reduction surgery;8 not surprisingly, further analysis showed that the excess mortality was largely confined to patients who would have been excluded based on the original entrance criteria. This is a matter of public record but was never acknowledged in published reports of the trial. Final 5-year NETT results showed that in patients with upper lobe emphysema, lung volume reduction surgery improved survival, increased exercise capacity, and improved quality of life.9 By the trial’s completion, however, the procedure’s reputation had been tarnished irreparably by bad publicity from the deaths attributable to the misguided changes to the original eligibility criteria.
Disadvantages of randomized trials
The NETT exemplifies many of the drawbacks of randomized trials in surgery, particularly the need to wait long periods while they are being conducted. During the 8 years in which the NETT was ongoing, the number of lung volume reduction operations declined, with the typical center performing fewer than 6 cases per year, on average. That limitation is certainly not conducive to the development of a new procedure for a disabling condition in patients with no ready alternative.
Other disadvantages of randomized trials in surgery are their considerable expense and the fact that they often are not generalizable and often are not appropriate. Moreover, when they are flawed, randomized trials propagate, sometimes for decades, misleading information that is nonetheless considered “authoritative.” For instance, lung cancer kills more men and women in the United States than the next three cancers combined, yet, on the basis of a flawed randomized trial,10 the American Cancer Society advises smokers to wait for symptoms before undergoing chest radiography, instead of recommending annual screening chest radiography. This is a major reason why two-thirds of lung cancer cases are discovered too late to save the patient.
‘Better to know nothing than to know what ain’t so’
Indeed, this potential for randomized clinical trials, when flawed, to propagate misleading information makes the perceived authoritativeness of randomized trials both an advantage and a disadvantage. As Berger and colleagues noted a few years ago, overuse of randomized trials for evaluating emerging operations could have led to the demise of heart transplantation, mechanical circulatory assist devices, cardiac valve procedures, coronary bypass grafting, and repair of congenital lesions.11
For this reason, one of our responsibilities when reading the literature and conducting studies is not just to answer unanswered questions but to question unquestioned answers. As 19th-century humorist Josh Billings put it, “It’s better to know nothing than to know what ain’t so.”
A PERSONAL PERSPECTIVE
In my view, health care providers should restrict the application of new procedures to a limited number of centers of excellence that have appropriate resources and experience. Those centers should be required to document and report specified information regarding morbidity, mortality, and objective measures of outcome; if they do not comply, they should lose the privilege of doing such research. The data should be reviewed by an independent, nongovernmental scientific panel. In this way, the procedure can be offered to appropriate patients, insurers and patients can be protected against abuse, and the necessary data can be collected for objective analysis.
Idea to implementation: A personal perspective on the development of laparoscopic nephrectomy
By Ralph V. Clayman, MD
Change in the surgical world involves three aspects, which I refer to as the three Ds: discovery, development, and dissemination. Change requires proof that the new method is superior to the old. When we, as innovators, develop something “new,” I believe that our immediate subsequent task is to do everything we can to prove that this “new” finding is of no value whatsoever before we determine that it is worth advancing.
Getting to Malcolm Gladwell’s tipping point—the act or event after which nothing is ever the same— requires a team of people, usually from different disciplines, coming together to concentrate on a problem, or an individual whose experiences in different fields provides the ability to “see” the next level. In my opinion, one person working in one discipline rarely leads to breakthrough progress in medicine.
These observations about surgical innovation stem largely from my experience in the development of laparoscopic nephrectomy, in which I was privileged to play a role while at Washington University in St. Louis, which I will outline here.
THE HISTORY BEHIND LAPAROSCOPIC NEPHRECTOMY
After doing preliminary work in dogs, the German surgeon Gustav Simon performed the first human nephrectomy in 1869, in a woman with a ureteral vaginal fistula. The operation was a success: it took him 50 minutes to complete the procedure, and 6 months later the patient went home.
From that point in 1869 until 1990, progress in nephrectomy was minimal, with open surgery remaining the gold standard. While the surgeon’s tools remained largely unchanged, the advances that did occur were in anesthesia, analgesia, and antibiotics, which allowed patients to better survive the onslaught of the operation.
In an unrelated arena, laparoscopy was developed in 1901 by another German surgeon, Georg Kelling, who pumped air into the peritoneal cavity of a dog in a successful effort to stop bleeding from the stomach. Within the pneumoperitoneum, Kelling was able to examine the canine organs with a cystoscope at pressures as high as 140 mm Hg. This discovery was not applied clinically, however, until 9 years later when the Swedish gastroenterologist H.C. Jacobeus used Kelling’s pneumoperitoneum concept and a cystoscope to visualize the peritoneal cavity to search for cancer. The technique advanced little in the subsequent decades —apart from Semm’s seminal laparoscopic removal of the appendix in the 1960s—until 1985, when the first laparoscopic gallbladder removal ignited the era of laparoscopic cholecystectomy.
Three technological developments spurred this recent surge in laparoscopy: (1) the ability to affix a camera to the endoscope, (2) the ability to display the camera’s images on a video screen, and (3) the development of self-feeding clip appliers to allow occlusion of vascular or ductal structures.
THE EVOLUTION OF LAPAROSCOPIC NEPHRECTOMY
Discovery
I became interested in the possibility of laparoscopic nephrectomy during the laparoscopic cholecystectomy craze in the late 1980s. At that time, I was working with Dr. Nat Soper, performing laparoscopic cholecystectomies in pigs to show that the procedure could be done safely with electrocautery rather than a laser. As it turns out, the anatomy of the porcine kidney is such that the colonic reflection lies medial rather than lateral to the organ. As such, the kidney is quite visible as soon as one enters the abdomen. Indeed, the kidney seemed to be saying to us, “Hey, what about me? I could come out through that hole too.” That is basically how the idea arose.
So, along with Dr. Lou Kavoussi and many others in our research team, we attempted laparoscopic nephrectomy in the pig and succeeded: the kidney could be removed through a small hole by entrapping it in a sack, breaking it up in the sack, and pulling it out.12 The team involved in this discovery were specialists in urology and general surgery as well as biomedical engineers from industry, specifically a team from Cook Urological led by Mr. Fred Roemer.
After performing this technique numerous times in the laboratory, we reduced the operation’s duration to 90 minutes, at which point we believed the procedure had advanced sufficiently to be considered for clinical use.
Development
The patient we selected for the initial clinical case was an 85-year-old woman with a 3-cm mass in her kidney. She was deemed to be “too sick to operate on,” so she was presented to me as a candidate for the new laparoscopic procedure.
Amazing as it may seem in our current medical climate, at that time (1990) we were faced with the question of whether or not to seek institutional review board (IRB) approval. The argument could be made that since radical nephrectomy had been practiced for 120 years and laparoscopy had been around for nearly 100 years, the combination of these two well-accepted procedures might require nothing more than physician-patient informed consent. However, the concept of “informed consent” in this context was problematic: what could we tell the patient about a procedure that had never been done before except that if it was not working out we would convert to the standard open procedure?
A senior colleague—actually my boss at that time, Dr. Bill Catalona—sagely advised me to get IRB approval, noting, “If the operation works out well, you’ll be fine, but if it doesn’t work out well, they’ll kill you if you don’t have approval.” So we fortunately ended up seeking (and receiving) IRB approval, as well as providing, as best we could, informed consent to the patient and her best friend.
Our next consideration was designating a team member to determine if and when conversion to open surgery would be necessary. We needed a “referee” to aid in objectively determining a point at which we should convert. For our team, that person was Dr. Teri Monk, our anesthesiologist, who had no previous experience with our laboratory work but understood what we were attempting.
So we proceeded with the first clinical laparoscopic nephrectomy on June 25, 1990. The kidney was embolized the morning of the procedure. Five laparoscopic ports were placed. The clip appliers proved too small for renal vein occlusion, so the main renal vein had to be traced to its branches; in total we clipped five separate sets of renal vascular branches. The kidney was ensnared and morcellated, which took 7 minutes. Total operative time was 6.8 hours. The complications that arose were not anticipated:
- Intraoperative oliguria due to the prolonged pneumoperitoneum
- Fluid overload (postoperative congestive heart failure) due to providing fluids to the patient as though this were an open procedure
- Dilutional anemia, again due to providing excessive fluids for a closed procedure.
Postoperative pain medications consisted of one dose of morphine sulfate. The patient was discharged on postoperative day 6 and resumed normal activities by postoperative day 10.13
Dissemination
Before a new procedure is disseminated, evidence of the four Es—efficiency, effectiveness, equanimity, and economy—must be obtained. In retrospective reviews, laparoscopic removal was associated with a slightly longer operating time but much less blood loss, a shorter hospital stay, and fewer complications. The immediate cancer cure rate was the same for open and laparoscopic nephrectomy, and over time the laparoscopic procedure has been shown to be just as good as open surgery at 5 and now 10 years. Also, with time, laparoscopic nephrectomy was shown to reduce institutional costs.
The next question was the proper way to disseminate this knowledge. At Washington University we took the traditional route of providing courses, offering 17 courses on laparoscopic surgery to nearly 1,000 urologists from 1985 to 2002. But as Winfield and associates later showed, only 54% of urologists who completed a 2.5-day hands-on, laboratory-based laparoscopic course actually ended up introducing laparoscopy into their practice.14
The challenge of dissemination is still with us, and we need to find better methods of transferring new skills to our surgical colleagues. In this regard, longer experiences, such as weeklong mini-fellowships and the development of procedure-specific surgical simulators, hold great promise.
UNANSWERED CHALLENGES, UNMET NEEDS
With the advent of any technology comes a cornucopia of unanswered questions and challenges. In the areas of discovery and development, a key question is whether every procedure performed using a new Food and Drug Administration (FDA)-approved technology requires a separate approval by the IRB and ethics committee. For instance, if robotic prostatectomy is approved and performed, are separate approvals needed for robotic nephrectomy, robotic pyeloplasty, and robotic vasectomy? Where would or should the approvals end?
With respect to dissemination, many questions remain: How is a new technology taught effectively? How is surgical competency tested? How is clinical performance or proficiency evaluated?
One problem specific to dissemination is a lack of funding. While ample funding is available for discovery and development, as they bring prestige and profit, dollars are scarce for dissemination, or the teaching and testing of competency and proficiency with new procedures.
Evidence of our failure to educate the postgraduate surgeon abounds in terms of poor outcomes and malpractice suits. The response of government all too often is the knee-jerk reaction to protect (ie, regulate), not educate. To be sure, we can do better, but only if our society commits to the process—not with words, but with funded educational action.
With regard to the last, I believe there is an unmet need for the development of accurate, validated surgical simulators. As a society, we need to find a way to fund the development of simulators for each surgical subspecialty and then use these devices to objectively test an individual surgeon’s manipulative skill as well as cognitive ability when he or she seeks certification or recertification—and perhaps, albeit in an abbreviated 5-minute format, before beginning each operative day. We owe this to ourselves, but most of all to our patients, who in all confidence place their lives in our hands.
Special perspectives in infants and children
By Thomas M. Krummel, MD
If we surgeons take a step back and consider for a moment what has changed in the operating room (OR) in the past 50 to 60 years, the clear answer is, “Just about everything.” The monitors, pumps, transport devices, and OR tables and lights have all changed dramatically, as have the tools, catheters, sutures, energy sources, scopes, staplers, ports, valves, and joints. If we consider technologies outside the OR that guide what we do inside the OR, the changes are just as striking. Circulatory assist devices for the failing heart and widespread use of dialysis for the failing kidney postdate 1950, as does all of our modern imaging capability—ultrasonography, computed tomography, magnetic resonance imaging, positron emission tomography, functional imaging. As for pharmacotherapy in 1950, there were three antibiotics, no antivirals, one antifungal, and three chemotherapeutic agents. Open drop ether was the anesthetic of choice. Not only have the tools and technologies changed, but virtually every procedure has been changed. Both our profession and the industry that has developed these devices and tools can be rightfully proud.
It is likewise necessary to recognize that our patients have been partners in this innovation. Many of them have given informed consent to participate in research and experimental procedures with the expectation that the benefits might accrue only to future patients and not to themselves. That is a hell of a contribution, and we can be proud of our patients’ partnership.
THE GOOD, THE BAD, AND THE UGLY OF INNOVATION
The history of progress in surgical care is always about innovation, and such progress almost always begins with an unsolved patient problem, regardless of the solution that is developed, be it a tool, a device, a technology, or a surgical procedure. At the same time, any discussion of the ethics of surgical innovation should recognize that while efforts to solve patient problems over the years have had many good results, they have also had some bad results and even the occasional ugly result.
This conference has already focused on much of the good that has come from surgical innovation, including transplantation, remarkable advances in cardiac and gastrointestinal surgery, a host of devices, and too many other benefits to list. Yet missteps have been made along the way, such as bloodletting, gastric freezing as a therapy for ulcer, and carotid denervation for treatment of asthma in children.
Then there are the ugly incidents, and these notably include a number of cases involving children, an issue of special interest to me as a pediatric surgeon. Consider the following examples:
- Edward Jenner’s notorious cowpox experiment in the late 18th century was conducted in an 8-year-old boy.
- A well-documented literature shows that orphans were used as subjects for tuberculosis and syphilis inoculations.
- The more recent case of Jesse Gelsinger involved a teenager with a nonlethal condition who died in a clinical trial of gene therapy, after which an undisclosed financial interest on the part of one of the treating physicians was revealed.
It should give us pause to note that many of these practices that look foolish in hindsight probably seemed more rational at the time they were undertaken.
CHILDREN: THE ORPHANS OF INNOVATION
Children have been the orphans of innovation, as technology development specifically for children has traditionally been a low priority. There are several reasons for this:
- FDA standards for approving therapies in children are high. For instance, the vast majority of chemotherapeutic drugs are not approved for use in children because conducting a trial specifically in children is deemed too expensive.
- Pediatric markets for therapies are small.
- The payor mix is poor.
The benefts of duality
Nevertheless, children have benefited enormously from the duality of technology development, in which a technology developed for one population—either adult or pediatric—ends up benefiting both populations. For instance, no one would have invented the pulse oximeter to care for a child, yet now it is the only device with which infants and children are monitored in the operating room and during transport.
Likewise, in some cases the solutions to pediatric problems have had reciprocal benefits in adults. Ligation of the patent ductus arteriosus and the Blalock-Taussig shunt for tetralogy of Fallot opened the door to our understanding of surgery on the great vessels and ultimately enabled the development of cardiac surgery. Similarly, the early impetus for Thomas Starzl’s groundbreaking work in transplantation was focused on children with biliary atresia even though this work is now much more widely applied in adults.
ETHICAL PRINCIPLES APPLY EQUALLY TO ADULTS AND CHILDREN
The principles of medical ethics that began with Hammurabi in 1750 BC and progressed through Hippocrates’ work circa 400 BC, the 1946 Nuremberg medical trial, the 1964 Declaration of Helsinki,15 Henry Beecher’s classic exposé in 1966,16 and the 1979 Belmont Report17 are just as valid for children as they are for adults.
Francis Moore, the great surgeon who created the environment and the team at Brigham and Women’s Hospital that facilitated the first twin-twin transplant, identified six important components of ethical surgical innovation:18,19
- A solid scientific background (basic laboratory research)
- A skilled and experienced team (“field strength,” as Moore called it)
- An ethical climate within the institution
- An open display for ongoing discussion
- Public evaluation
- Public and professional discussion.
The principles behind these components remain as true today as they were 20 years ago when Moore outlined them.
SPECIAL CONSIDERATIONS IN PEDIATRIC SURGERY: A CASE STUDY IN MATERNAL-FETAL MEDICINE
The Belmont Report, mentioned above, was developed by the US government in 1979 to form the basis of regulations for federally funded research involving human subjects.17 The report identified three basic principles that must underlie such research:
- Respect for persons—protecting the autonomy of all subjects, treating them with courtesy, and allowing for informed consent
- Beneficence—maximizing benefits from the research initiative while minimizing risks to the subjects
- Justice—ensuring reasonable, nonexploitative, and well-considered procedures that are administered fairly.
In pediatric surgery, everyone agrees that the “best interests of the child” must be protected, but the issue of autonomy (a key element of the first Belmont principle) is more difficult to define, of course, when the patient is a child rather than an adult. The question of autonomy is especially tricky in the evolving field of maternal-fetal medicine: what if the patient is a fetus and the mother is an innocent bystander?
Over the past 20 years, tremendous progress has been made in our understanding of diseases of the fetus, particularly diseases that limit fetal viability and diseases that cause serious organ damage but which may be more responsive to postnatal therapy if they are treated prenatally. Michael Harrison, N. Scott Adzick, and a few of their disciples have laid the ethical groundwork for consideration of the fetus as a patient.
Considerations in maternal-fetal medicine
I will conclude with a case in maternal-fetal medicine for us to consider and perhaps debate in the panel discussion at the end of this session. As you consider this case, keep in mind several important observations relating to maternal-fetal medicine:
- The mother’s health interests cannot be underestimated.
- Most “fixable” fetal lesions (ie, those that interfere with development and cannot be fixed postnatally, but for which intervention in utero may result in normal development) are very rare. They include obstructive uropathy, lung lesions causing hydrops, congenital diaphragmatic hernia, sacrococcygeal teratoma, hydrocephalus, twin-twin transfusion syndrome, congenital high airway obstruction, hydrothorax, myelomeningocele, and congenital heart disease.
- The field is evolving, and the efficacy of therapy is supported by variable level I, II, and III evidence.
- The law has not kept (and perhaps cannot keep) pace with developments in this field.
Case study
A 24-year-old healthy woman has a fetus of 28 weeks’ gestational age with progressive lower urinary tract obstruction with megacystis, bilateral hydronephrosis, and oligohydramnios. In other words, there is diminished volume in the uterine cavity that causes compression of the fetal chest and subsequent respiratory compromise that will be fatal if not addressed. The karyotype is a normal 46,XY male. Serial urine sampling reveals electrolyte and protein profiles with a good prognosis.
Prenatal counseling with fetal therapy specialists suggests that this is the “perfect case” for a vesicoamniotic shunt. This is the least invasive, most successful fetal surgical intervention. It is done under local anesthesia and involves transabdominal transuterine percutaneous placement of a double-lumen pigtail catheter in the fetal bladder. There has never been a reported maternal death, and morbidities have been minimal. Renal and pulmonary function both are improved by approximately 80% in fetuses treated with this intervention, and survival is improved.
The father is eager to proceed. The mother is ambivalent. Should the mother be pressured to proceed, for the good of the child?
Questions to ponder
The following questions are intended to be provocative, with no clear-cut answers:
- Should (or does) the fetus have independent moral status? Is it full, graded, or none? Does it matter?
- What are the beneficence-based obligations to the fetus? At 28 weeks’ gestation, the fetus is viable outside the uterus. The fetus is otherwise well, without a lethal karyotype, and has currently good renal function.
- What are the beneficence-based and autonomy-based obligations to the mother? What are the mother’s obligations to the fetus?
- What if the mother ultimately decides to proceed and the insurance company denies coverage? What are the social responsibilities to care, cost, and research?
These questions lend themselves to discussion. As much as we surgeons like to be certain about what we do, we would do well to heed the quote from Voltaire that the great surgeon Norman Shumway hung on his office door: “Doubt is not a very agreeable state, but certainty is a ridiculous one.”
Bariatric surgery: What role for ethics as established procedures approach new frontiers?
By Philip R. Schauer, MD
Obesity is a staggering problem: 100 million Americans are overweight, 85 million more are obese, and another 15 million are morbidly obese (ie, ≥ 100 lbs above ideal body weight). The incidence of obesity is rising rapidly and threatens to shorten the life spans of today’s young generations relative to their parents. Unlike other conditions, such as cardiovascular disease and cancer, obesity has seen no widespread progress in management in recent years.
Recognition of obesity as a medical problem is a challenge in itself. Many people consider obesity to be a character flaw or a behavioral issue and fail to recognize it as a disease entity. Yet obesity is the root cause of many metabolic conditions and diseases with metabolic components, including type 2 diabetes, heart disease, blood pressure, metabolic syndrome, acid reflux, gout, arthritis, and sleep apnea.
The approach to obesity treatment can be conceptualized as a pyramid, with the aggressiveness of the intervention based on the patient’s body mass index (BMI). At the base of the pyramid, for patients with lower BMIs, are minimally invasive (and minimally effective) interventions involving changes in diet, physical activity, and other lifestyle factors. As BMI increases, so does the intensity of treatment, to include pharmacotherapy and eventually bariatric surgery. Traditionally, surgery has been considered only at the very top of the pyramid, for morbidly obese patients, and is usually not offered as an option for the vast majority of people with this condition.
The sad reality is that the various combinations of these therapies are effective in fewer than 1% of the approximately 100 million Americans who are obese. Because surgery has been shown to be the most effective therapy for obesity, the remainder of my discussion will focus on surgery, with an eye toward potential new indications for bariatric procedures and the questions they raise.
SURGICAL APPROACHES TO OBESITY
Bariatric surgery has evolved over the past 50 years. Although there are about a dozen different permutations of bariatric procedures performed in the United States today, they fall into one of three major types of operations, as outlined below:
Gastric banding reduces appetite and satiety by adjusting and tightening the gastric band. This procedure has been in existence for 10 to 15 years and represents about 25% of operations for obesity in the United States.
The biliopancreatic diversion procedure diverts most of the small bowel and radically reduces absorption of calories. Patients undergoing this procedure lose weight because few calories are absorbed into the body. This approach, while quite effective, is somewhat radical and represents only about 2% of the operations for obesity in the United States.
The Roux-en-Y gastric bypass procedure has been the dominant procedure over the past 15 to 20 years. A combination of the above two procedures, it involves reducing the gastric reservoir and bypassing the stomach and upper intestine. The reduction in gastric volume reduces calorie intake by enhancing satiety, and the limited foregut bypass moderately reduces absorption.
No randomized trials, but much support from observational studies
Virtually none of these procedures evolved with randomized controlled trals. Instead, they evolved incrementally, primarily on the basis of knowledge gained from case procedures. Despite the lack of randomized trials, these operations have been shown to be effective, particularly in patients with multiple metabolic abnormalities associated with severe obesity. A large body of data from case-control and cohort studies demonstrates not only dramatic improvement in metabolic abnormalities with the use of various bariatric procedures, but also improvements in quality of life and survival.20–26 The two most recent of these studies, published in 2007, found reductions in mortality of 29% (adjusted) and 40% among surgical patients compared with well-matched obese controls during mean follow-up of more than 7 years.25,26 Reductions in the incidence of cardiovascular mortality and, secondly, cancer-related mortality were the two major contributors to the overall mortality reduction in these two studies. Consistent with this latter finding, obesity is starting to be thought of as a disease that may lead to cancer.
NEW FRONTIERS FOR BARIATRIC PROCEDURES
The current indications for bariatric surgery have existed intact for about 25 years, and were based on limited evidence available at the time. They are basically as follows, assuming acceptable operative risk and appropriate patient expectations:
- BMI greater than 40 kg/m2
- BMI greater than 35 kg/m2 with significant obesity-related comorbidities.
Payors adhere strictly to these indications, such that they will not pay for bariatric surgery in a patient with a BMI less than 35 kg/m2. This raises questions about the appropriateness of such a firm threshold and whether expansion of these strict indications may be reasonable.
Even without broadened indications, the volume of bariatric procedures in the United States has grown dramatically in recent years. Whereas only 10,000 to 20,000 of these operations were performed annually in the 1990s, approximately 200,000 such procedures were performed in 2007, and this number is expected to double over the next 5 years or so.
This growth in volume has been paralleled by burgeoning media interest in bariatric procedures, particularly in the last few years. More attention can be expected as we increasingly recognize the potential of bariatric procedures for indications beyond strictly the treatment of morbid obesity. At least two new frontiers loom: metabolic surgery and endoscopic surgery.
Metabolic surgery
Procedures that incorporate a bypass—the Roux-en-Y gastric bypass and the biliopancreatic diversion —have been associated with a reversal of metabolic diseases such as type 2 diabetes.27–32 Many patients with type 2 diabetes who have undergone these procedures have been able to be weaned off insulin and insulin-sensitizing medications while maintaining normal blood glucose levels. The effect has been profound and immediate, occurring even before the patient loses weight. In one series of patients with type 2 diabetes who had undergone a bypass operation, 30% left the hospital in a euglycemic state.29
These observations have been made primarily in the morbidly obese population, who are the primary candidates for bariatric bypass procedures. However, because of the rapid improvement in metabolic abnormalities that has been observed, interest has arisen in applying these procedures to populations that are not morbidly obese. Bypassing of the foregut appears to be critical, perhaps because it tempers the release of hormonally active peptides from the gastrointestinal tract.33 In any case, the gut is regaining recognition as a major metabolic organ.
In light of these hypotheses, the duodenal-jejunal bypass is a bariatric procedure that may be beneficial for a patient with type 2 diabetes who is not morbidly obese. In this operation, the stomach volume is preserved but the foregut is bypassed. In a small experimental series from Brazil, patients with type 2 diabetes who were normal weight or only slightly overweight had resolution of their diabetes following this procedure, without any weight loss.34
New applications for endoscopy
Another area of development is endoluminal and transgastric bariatric surgery. Endoluminal surgery is performed entirely within the lumen of the gastrointestinal tract using flexible endoscopy. Transgastric surgery is performed within the peritoneal cavity, which is accessed via a hollow viscus. Both approaches use natural orifices to gain surgical access, thereby avoiding access incisions and scars.35
The benefits of such an approach are numerous:
(1) fewer complications and side effects; (2) less invasiveness, and thus the ability to perform in the outpatient setting; (3) reduced procedure costs; and (4) better access to treatment. The implication in terms of indications is the potential to use such procedures to prevent progression to morbid obesity.
Examples of these procedures are proliferating:
Gastrojejunostomy reduction is an endoscopic procedure that involves reducing the dilated opening of the gastric pouch after gastric bypass surgery. New endoscopic suturing or stapling devices enable the outlet reduction without requiring surgery. The result is enhancement of weight loss without a major operation.
Endoluminal suturing uses endoscopic instruments to suture the stomach to reduce its volume. When this procedure is perfected, the patient should be able to leave the endoscopy suite and return home within a few hours.
The duodenal sleeve is an avant garde concept in which an internal sleeve is threaded into the stomach and down the intestines.36 The sleeve covers the absorptive surface of the small bowel, preventing absorption of nutrients to cause weight loss. This procedure has been shown to have a strong antidiabetic effect as well.
Clinical applications of these operations are emerging. An endoluminal sutured gastroplasty procedure to shrink stomach volume has been shown in a small clinical trial to cause loss of significant excess body weight; the operation leaves no scars and is associated with a low risk of bleeding or any type of surgical complication.37 A similar procedure is in development that involves staples instead of sutures.
How best to validate innovations moving forward?
As we move into these new eras of metabolic surgery and endoluminal and transgastric bariatric surgery, interesting questions arise. We as innovators and caregivers are ethically obligated to demonstrate reasonable safety and efficacy before such new procedures are performed widely. Although some of these emerging procedures involve new devices that will go through the FDA review process, many are existing procedures for which indications may be expanded, while others are permutations of existing procedures for which no formal rules for validation exist. For new procedures that differ substantially from existing proven procedures but which do not require new devices, should we not be ethically bound to demonstrate safety and efficacy even though they do not require FDA review? These are the challenges that await as innovation takes bariatric surgery to new frontiers.
Natural orifice transluminal endoscopic surgery: Too much too soon?
By Christopher Thompson, MD, MHES
Although the endoscope has changed very little since the first fiberscope was developed 50 years ago, the accessories and other instruments used in conjunction with the endoscope have changed remarkably. These include clips for hemostasis, ultrasonographic technology, and instruments for tissue dissection.
These advances in endoscopy, combined with advances in laparoscopic surgery, have led to the convergence of these two fields, culminating in the new field of natural orifice transluminal endoscopic surgery (NOTES). In NOTES, the surgeon enters a natural orifice and punctures through a viscus to perform surgery, removes the endoscope, and closes the area without leaving a scar.
HISTORY OF NOTES AT A GLANCE
NOTES was patented as a concept in 1992. Its first application was as an exploratory procedure in the pig in 2004.38 Soon thereafter, therapeutic NOTES procedures in animals were reported, including tubal ligation, organ resection, cholecystectomy, and splenectomy.
Particularly notable in the development of NOTES is the extremely short interval between early animal experiments (2004) and the first human procedures, which took place as early as 2005 when surgeons in India used the technique to perform a human appendectomy. Since then, more than 300 NOTES procedures have been performed in humans throughout the United States, Europe, Latin America, and Asia, for applications ranging from percutaneous endoscopic gastrostomy rescue to transvaginal cholecystectomy.
This rapid adoption of NOTES in humans is concerning, as it raises clear questions about whether there has been time for adequate oversight and safety assessment. For instance, at a surgical conference in April 2008, questions and debate swirled around whether a large Brazilian registry of more than 200 NOTES cases did or did not include two deaths. Other ethical issues raised by NOTES are discussed further below.
DRIVING FORCES BEHIND NOTES
The medical rationale
Abdominal wounds can cause pain, are unaesthetic, and are prone to wound infections, ruptures, and hernias. They sometimes cause adhesions or may lead to abdominal wall syndromes with scar neuromas that cause pain later. They also require general anesthesia. Beyond these shortcomings of incision-based procedures, NOTES offers potential reductions in length of stay and therefore in cost. Moreover, certain patient populations may specifically stand to benefit from NOTES, such as obese patients, those with abdominal mesh in place, and those undergoing palliative procedures. This is the essence of the medical rationale for NOTES, which is somewhat thin.
Professional organizations and courses
In July 2005, leaders from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons convened a working group to support and plan for the responsible development of NOTES.39 The group formed the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR), an organization that has since sponsored several conferences on NOTES and procured millions of dollars in grants for NOTES research in animals. (In the interest of full disclosure, I am one of the founding members of NOSCAR.)
Additionally, leading institutions in this field have held numerous hands-on courses on NOTES throughout the United States, Europe, Latin America, and Asia. These courses, including those held by my laboratory at Harvard University, are designed to teach colleagues at other institutions how to set up an appropriate animal laboratory and to promote and encourage proper research in NOTES. There have been unintended consequences, however, as we have learned that some course attendees have returned to their home countries and immediately started using the techniques in humans.
New technology
At the July 2005 working group meeting that launched NOSCAR, we determined that several technological advances were needed before NOTES could be safely applied to humans. These included development of multitasking platforms, better devices for tissue apposition and fixation, better imaging and spatial orientation, and improved means of retraction.39 Industry responded with novel devices and end effectors such as guide tubes, direct drive systems, endoscopic suturing devices, magnetic retraction, devices for closing luminal defects, flexible staplers, and computerized robotics.
Other driving forces
Additional forces have undoubtedly contributed to the rapid development of NOTES:
- The slowdown in innovation in general surgery in recent years has left a vacuum to be filled.
- An abundance of venture capital has been available to rush into that vacuum.
- Perceived patient demand (owing to cosmetic advantages) has been a driver, especially in cities such as Rio de Janeiro, Milan, and New York.
- The fear of being left behind is a factor that cannot be underestimated. Surgeons who failed to convert to laparoscopic techniques from open techniques in the early 1990s for procedures such as cholecystectomy, fundoplication, and splenectomy were losing their patient bases. Many surgeons fear a similar phenomenon today if they do not adopt NOTES into their practices.
ETHICAL ISSUES RAISED BY NOTES
As NOTES moves toward further evaluation in humans, several ethical questions need to be grappled with:
- Must there be a significant potential for improvement in care before an innovation advances to human research?
- Is the cosmetic benefit of NOTES sufficient, considering the substantially increased risk? For instance, laparoscopic cholecystectomy is well established, whereas NOTES cholecystectomy carries an increased risk of bile duct injuries and other injuries. Is NOTES worth the risk?
- What about the corporate agenda behind new technologies and its associated influence on the media?
- Are hospital IRBs adequate to the task of evaluating and monitoring these questions, and will they be independent of the impact of hospitals’ larger agendas?
Finally, the problem of premature adoption of this technology is particularly concerning. I heard a surgeon explain at a course that he performed NOTES on a few pigs at a previous course and then returned home to Peru and immediately started performing it on patients at his ambulatory surgery center. There is also the temptation for well-respected surgeons to go to other countries to practice their NOTES skills before returning to the United States, in hopes that their experience will help them attain IRB approval. Practices like these raise questions about what ethical responsibilities lie with those of us who have pioneered the technology and are trying to develop and disseminate it responsibly. We can try to vigilantly watch course attendees from certain countries, but there is little we can do in the absence of regulation and enforcement in those countries. These are difficult ethical challenges.
Panel discussion
Moderated by Jonathan D. Moreno, PhD
Dr. Jonathan Moreno: I would like to begin with any questions that the panelists have for one another.
Dr. Philip Schauer: I would be curious to hear how my colleagues define incremental changes in a procedure. In other words, what constitutes a new procedure versus a modification of an existing one?
In bariatric surgery we are grappling with a procedure called the sleeve gastrectomy, which poses challenges comparable to lung volume reduction surgery as described by Dr. Cooper. Many of us believe that this procedure is just a slight modification of a gastroplasty, yet payors consider it an entirely different procedure, and some want 5 to 10 years of follow-up data before they will pay for the operation.
Dr. Joel Cooper: That is not an easy question, but I would approach it from the standpoint of what you would tell the patient. When we were first developing lung volume reduction, we performed it only in patients who had absolutely no other alternative. Only later in its development did we offer it as an alternative to transplantation. How do you approach the patient when you can already achieve a very good result with an existing procedure and you can tell the patient, with some assurance, what to expect with that procedure? In the case of NOTES, I do not think that the cosmetics are sufficient justification.
The second aspect is regulatory. I am not a supporter of the FDA’s practices for the introduction of new procedures, but I believe strongly that universities have been derelict in setting the standard for the introduction of new procedures, particularly minimally invasive procedures. They have been using these procedures as marketing tools to vie with private hospitals for dollars and patients. I cannot say whether the rapid promulgation of these procedures at too early a stage actually can be prevented, but I do not recall the chairmen of major surgery departments getting together to issue public statements about the proper protocol for introducing new techniques. As Pogo said, “We have met the enemy and he is us.”
This may not answer your question, but I believe there should be no payment for any new or novel procedure for a certain period after its introduction, and certainly the hospitals should not be able to profit from it, although the expenses of a new procedure may be recouped. That alone would perhaps put the brake on some of the marketing and the financial incentives, and it might separate, to some degree, the development of new procedures from economic interests.
WHO SHOULD OBTAIN INFORMED CONSENT?
Dr. Moreno: Should informed consent be obtained only by a knowledgeable third party rather than the surgeon-innovator?
Dr. Thomas Krummel: The question is whether there is a disinterested third party who truly is knowledgeable; in cases where there is such a person, I see no downside to having that person involved. However, the notion of having someone who is not associated with clinicians or surgeons obtaining informed consent makes me uncomfortable. Informed consent is not a piece of paper. It is a trust between physician and patient, and to ignore that could leave you in a heap of trouble.
Dr. Cooper: I agree, but another process is important as well. In proposing lung transplantation before there had been any successful transplants, we defined in advance the standards, indications, and contraindications that we thought should apply. We did this in the absence of any particular patient, and it relieved us of the difficulty of making arbitrary decisions that may have led to unfairly accommodating one patient over another. Once the standards have been set in this way, they can be applied—whether by the investigator or by a committee—in an objective way to the group of patients that is most appropriate in the early phases of development.
Dr. Ralph Clayman: It is difficult for the inventors of an operation to dampen their enthusiasm for their creation to a point where they are as objective as they should be. Joel is bringing up situations for which there are no alternatives. My realm is an area in which there were well-established alternatives for everything we have done laparoscopically or percutaneously, and it was difficult to decide the indications or contraindications early on. Often, the early indications only had to clear the threshold of not seeming ridiculous.
The early development of percutaneous stone removal at the University of Minnesota took place entirely outside the purview of an IRB. Percutaneous nephroscopy had been around since 1955, and we extended it to plucking out a stone. That is how that entire field developed. Early on, we were not going to go after a stone that was as big as a fist because we did not have a way to break it up. As time went by, however, it evolved to the point where there was no stone in the kidney—regardless of its size, location, or hardness— that could not be removed through a small hole in the back. But that entire evolution proceeded without IRB approval.
For laparoscopic nephrectomy, for which there were well-known alternatives, who should have obtained the informed consent? Should it have been me, bringing along the “white coat” factor and not being able to really explain the potential problems since nobody had yet gone there, despite my rapport with the patient? Or should it have been a third party with whom I had discussed the procedure and its possible problems? I do not know the answer, but it raises an interesting point, especially in this age of IRBs and ethics committees.
Dr. Krummel: It is not unlike what we have tried at Stanford when we are not sure of the boundary for IRB consultation. The surgical chairs are willing to convene and essentially police one another, so that when the neurosurgeon proposes a brain transplant, there probably will be a pretty interesting conversation before it gets the green light.
Dr. Cooper: My experiences with IRB involvement differed quite a bit between my work in lung transplantation and my work in volume reduction surgery, but the differences owe a lot to the countries where I was practicing at the time. I did my early work in transplantation in Canada, where I did ask for approval from my hospital’s ethics committee and other relevant committees. In Canada, the hospital had a global budget, and it made a decision that it was willing to use part of its budget for transplantation. We received no fees for years, until the operation was proven to be effective, but that did not stop us from developing the procedure.
I had returned to the United States when I began my work with lung volume reduction, and I did not ask the IRB for permission to do that procedure. My justification was that, theoretically, volume reduction was similar to accepted practices for removal of nonfunctioning lung to improve respiratory mechanics (bullectomy) and that we would simply be applying the concept to a different group of patients. However, unlike in Canada, I did not have institutional financial support for doing this new procedure, so how was I going to do it if the hospital could not receive payment for it? I went to the IRB, but instead of asking for permission to do the procedure I asked for permission to study the procedure and to collect data on it. In that way, I was notifying the IRB of my action and thus giving it an opportunity to act. If I had gone to the IRB to approve the procedure, however, the operation would have been labeled experimental by insurance companies, who would have then found a way to deny payment. At least that was how it was in those days.
MARKETING OF MEDICINE: IS THERE NO TURNING BACK?
Question from audience: What makes you think that in 10 years there won’t be 100 million obese Americans watching television ads for noninvasive bariatric surgery promising to rid them of their obesity problem? What will keep that from happening?
Dr. Krummel: Nothing. What makes you think it is not happening now? Just look at the ads for the Lap-Band in the lay press.
Dr. Clayman: We already have direct marketing of drugs and direct marketing of facilities. What Joel said is true: “the enemy is us.” When I was in training, the idea that a physician would advertise was considered unethical. I still consider it thus. But everybody is doing it, so should that make it acceptable? I think not.
The same thing is true of the huge amount of money spent marketing drugs on television. Why should a single nickel be spent to advertise health care beyond generically informing the public of important health care issues and initiatives? You cannot go to an airport without seeing a surgical robotics program being advertised or a hospital being advertised. You cannot turn on National Public Radio without hearing well-financed spots touting the achievements of a hospital. You cannot watch television without seeing ads for erectile dysfunction medications or other new drugs. It is a waste of dollars. If we took all of that money and redirected it, we could probably solve much of the indigent health care problem, but we as a society have chosen not to do that.
SHOULD THE BAR BE RAISED FOR SURGICAL TRIALS?
Dr. Moreno: Let’s consider some additional questions. Why shouldn’t the government raise the bar on the level of evidence needed to gain regulatory approval for new devices? Why not require randomized trials, as is done for drugs?
Dr. Cooper: Procedures that lend themselves to a randomized trial should be studied at a limited number of centers with mandatory reporting and preset indications for promulgation and payment. I believe that universities have been derelict in their duty to require this level of evidence.
This question is always nuanced, however. Consider the case of laparoscopic procedures. They offer the advantage of smaller incisions, yet how many patients have had to die or suffer serious consequences for the sake of these smaller incisions? On the other hand, how many patients may have been saved from pulmonary embolism, wound infections, or a prolonged hospital stay as a result of laparoscopic techniques? Only a randomized trial could demonstrate whether or not there has been an overall payback from new procedures such as this, although even then the payback may be present for some types of patients but not others.
Dr. Schauer: The problem is expense. Perhaps it is all a matter of economics. Return on investment for the drug industry is something like 10 to 1, but return on investment for the medical device industry is generally much lower. Therefore, conducting large randomized controlled trials is extremely expensive and much more complicated for a device or procedure. This may explain why the standard for trials is different for the two industries.
Dr. Krummel: Virtually all fetal surgical procedures have been subjected to a trial, several of them randomized. The National Institutes of Health paid for many of these trials. One such study prevented rapid uptake of the congenital diaphragmatic hernia operation, which has never been proven in a randomized trial to be better than our current therapy. It is a good example of a randomized trial making a difference.
Dr. Clayman: As Joel pointed out, surgery is constantly evolving, whereas a drug remains unchanged throughout its lifespan. If we had started a prospective randomized trial after we had done our first laparoscopic nephrectomy, the procedure would have died because we were not nearly as facile with our first 10 as we were after our first 100. The technology continues to develop, and the surgeon continues to develop his or her skills, which makes a study of this nature overly dynamic. Perhaps the best you can do is a retrospective, matched, controlled study with the same surgeon, comparing his or her results after 40 or 50 laparoscopic procedures with results after his or her 50 most recent open procedures.
Dr. Cooper: How do you put a brake on the system? Would some sort of limited trial perhaps put a brake on the too-rapid promulgation that we often see?
Dr. Clayman: In the general surgery realm, laparoscopic cholecystectomy came out of private practice. It did not come out of the university with its faculty and laboratories dedicated to exploration and investigation. It never was properly vetted in the scientific realm but rather came to the light of day as an “economic” edge.
Dr. Krummel: I would not underestimate the talent and creativity of those that we train who go out into private practice. Much innovation has come from very active practitioners.
Dr. Clayman: Right, but they do not have the infrastructure that we are blessed with at universities both to create and to validate.
Dr. Schauer: I agree that academia does not have a monopoly on creative ideas. But perhaps academia should play a major role in defining validation-type studies. That is one area where we may be especially well suited to meet an important need.
THE INNOVATION-TRAINING INTERFACE
Question from audience: I have a dilemma as a residency program director. Our residents want to learn the new technology—laparoscopic surgery, robotic surgery, etc—but we have them in our program for such a limited time. How do you justify teaching them new technology and at the same time still teach them basic, traditional surgical procedures, especially with the reduction in residents’ hours? It is fine to be able to do a nephrectomy laparoscopically, but if you get in trouble, you still have to know how to do a good open nephrectomy. How do you address this?
Dr. Schauer: I think the answer largely is fellowship training. Emerging procedures probably should be introduced in fellowship programs until they reach the point where they are so standardized that they become a major part of practice. For example, cholecystectomy quickly became part of general surgery practice, but laparoscopic colectomy took several years to evolve and was taught primarily in fellowship or advanced training, after which it gradually filtered down to residency programs.
Dr. Krummel: All of us who are responsible for training are wrestling with this problem. Residents are expected to learn more yet do so in less time. One approach would be early specialization, so that instead of 5 years of general surgery, you would have 3 years of general surgery and then 3 years of, for instance, thoracic surgery. Also, Ralph mentioned earlier the advantage of skills labs. We increasingly see that type of approach as a backbone for providing broad training without putting patients at harm.
As for teaching the use of new technology, first you have to teach the existing base of practicing surgeons. Here again there is much to be said for skills labs, and I give credit to the American College of Surgeons for its drive to establish and accredit centers around the country as a way to teach this base of surgeons.
Dr. Schauer: If I may expand this question beyond residency and fellowship training, how do we balance the desire to share new innovations with our colleagues against the need to temper their desire to prematurely jump into an area where they do not yet really belong? Chris, I know this applies to your challenges in disseminating knowledge about NOTES.
Dr. Christopher Thompson: Yes, courses on NOTES are also being held in conjunction with all the major society meetings, and we are seeing many enthusiastic trainees at these hands-on courses. The original intent was to give attendees instruction on setting up their own animal labs, yet some trainees took it beyond this limited purpose. As a result, some in our field believe that we should not allow foreign physicians to come here to be trained in NOTES, for fear they will go back to their home countries and use it on humans. I am not certain that that approach is the best way to go, but there has been much discussion about how to handle this. It is a real conundrum. Certainly there are a number of surgical residents and gastroenterology fellows who are clamoring to get into the lab right now and learn these techniques.
Dr. Clayman: This goes back to our earlier discussion about from where new technologies should emerge. What frightens me are the consequences of creative activity occurring outside the university, where there are no laboratories or animal or cadaver models for refining or testing a technique. To me, it was frightening to see laparoscopic cholecystectomy suddenly emerge as a craze without the proper animal and clinical studies having been done. That is not the way I believe clinical research should go forward. I once heard a prominent urologic surgeon say at a major surgical meeting, after a presentation on the impact of percutaneous stone surgery on the canine kidney, “Now that I’ve done a thousand of these in humans, it’s reassuring to know that it’s safe to do in dogs.” That is not the way it should happen, and every time it does happen that way, we pay a large price, some of us as individuals and all of us as a society.
Dr. Cooper: The answer therefore is to use our academic facilities to facilitate the training of those in community practice. We should continue to offer training because we have the resources to make it available.
Dr. Clayman: Yes, and this is why I emphasized earlier that support for surgical training centers is so essential. I see all the dollars spent on health care advertising and wonder why these dollars are not instead poured into surgical training, or research facilities, or training simulators.
The way we should train surgeons in new technologies is to train them on simulators equipped with a properly vetted curriculum. This is the future for training, because once you put instruments through small ports, everything becomes measurable—economy of motion, past pointing, and efficiency; simulators with a curriculum will also be able to assess the trainee’s cognitive abilities. When an individual performs well on the simulator, he or she can then come into the operating room and work with surgeons experienced in the procedure. The use of simulators in this manner should ultimately improve the overall quality and safety of each surgical specialty.
RISE OF THE ROBOTS
Question from audience: I am curious how the panel members interpret early randomized trial data showing an increased cost without an improvement in care with the use of surgical robots in certain procedures. Should we persist or consider an investment in the future as robotic technology improves and surgeons further adapt to it?
Dr. Cooper: I think the robot should be used only for those procedures for which it has unique capability and can perform a task better than we can. It appears that the robot performs better than the ear, nose, and throat surgeon for operations on the base of the tongue. The same may be true of prostate surgery, but I am not certain. But to do a laparoscopic Nissen repair with a robot…as Dr. Nat Soper of Northwestern University has said, “If I needed a robot, I shouldn’t be doing laparoscopic Nissens.”
The robot provides light, it gives you magnification, and it reduces tremor. We should concentrate on its use for operations where these attributes are particularly valuable. But we should be wary of its use as an expensive marketing tool.
Dr. Clayman: The robot provides you with superhuman capabilities: 10 to 30X magnification, no tremor, a 540-degree wrist, instrumentation with 6 degrees of freedom, and motion scaling. It allows you to be a better surgeon than you are without it. I agree that it is expensive. It is woefully overpriced at this point, but I believe the expense will come down with time. It is no different than the first computers, which were terribly expensive. The robot enables surgeons to do a better job than they would without it if we are talking about reconstructive-type surgery.
Ergonomically, the robot is very positive for the surgeon. For the first time, the surgeon is actually allowed to sit down in a comfortable environment and can work for 4 hours straight, get up at the end of the surgery, and feel fine. If you are older than 50 and you operate standing at the table staring at a television screen on the other side for 4 to 6 hours, you are going to ache afterwards. I believe surgeons work better if they are comfortable.
Dr. Schauer: At least within my field of general surgery, there has been no evidence that this superhuman ability has translated into superhuman results, in terms of reduced operating time, fewer complications, or better efficacy. We should probably develop the metrics to measure progress. How do the theoretical benefits translate into clinical benefit?
Dr. Clayman: It is not theoretical in radical prostatectomy if you look at the data. The potency rates for patients who undergo robotic surgery for these procedures are now almost 90%, which is something that no surgeon performing open prostatectomy has ever achieved. Fortunately, the continence mechanism is so strong in most adults that it does not matter whether prostatectomy is done with a robot or open surgery—patients are probably going to be all right. But the bottom line is that robotic surgery is a bit better. Most surgeons would use it if it were free. The problem is that it is so expensive right now and it is breaking the backs of many hospitals.
Dr. Schauer: You make a good point. Demonstrating metrics is important, and prostatectomy is a good example. But I am not aware of any other procedures for which benefit from robotic surgery has been documented.
Dr. Krummel: The history of robotic surgery is so interesting because the killer application was supposed to be coronary work—percutaneous bypass surgery. But then the heart port went to pot and patients with anterior wall lesions ended up not being a big enough group. It turns out that it is still difficult to do and there is not a lot of room. So prostatectomy has ended up as the initial killer application.
Keep in mind that the current robot is not an end device. We will see more. There are now robotic steerable catheters that I think will be adopted into NOTES procedures. This theme of immediate benefit versus follow-on iterations is the story of device development in this country.
- Gorman C. Are surgeons too creative? Time 1995; 146(Sept 4):56–57.
- Majeed AW, Troy G, Nicholl JP, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet 1996; 347:989–994.
- Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996; 347:984–985.
- Reeves B. Health-technology assessment in surgery. Lancet 1999; 353(suppl 1):3–5.
- Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Allen KB, Dowling RD, Fudge TL, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med 1999; 341:1029–1036.
- The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999; 116:1750–1761.
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345:1075–1083.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med 1986; 28:746–750.
- Berger RL, Celli BR, Meneghetti AL, et al. Limitations of randomized clinical trials for evaluating emerging operations: the case of lung volume reduction surgery. Ann Thorac Surg 2001; 72:649–657.
- Clayman RV, Kavoussi LR, Long SR, Dierks SM, Meretyk S, Soper NJ. Laparoscopic nephrectomy: initial report of pelviscopic organ ablation in the pig. J Endourol 1990; 4:247–252.
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991; 146:278–282.
- Colegrove PM, Winfield HN, Donovan JF Jr, See WA. Laparoscopic practice patterns among North American urologists 5 years after formal training. J Urol 1999; 161:881–886.
- Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2(5402):177.
- Beecher HK. Ethics and clinical research. N Engl J Med 1966; 274:1354–1360.
- The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: Department of Health, Education, and Welfare; April 18, 1979. Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.htm. Accessed July 28, 2008.
- Moore FD. Three ethical revolutions: ancient assumptions remodeled under pressure of transplantation. Transplant Proc 1988; 20(suppl 1):1061–1067.
- Moore FD. The desperate case: CARE (costs, applicability, research, ethics). JAMA 1989; 261:1483–1484.
- MacDonald KG Jr, Long SD, Swansons MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997; 1:213–220.
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004; 199:543–551.
- Christou NV, Sampalis JS, Lieberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004; 240:416–423.
- Sowemimo OA, Yood SM, Courtney J, et al. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis 2007; 3:73–77.
- O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006; 16:1032–1040.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222:339–350.
- Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 2005; 9:1112–1116.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003; 237:751–756.
- Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis 2006; 2:401–404.
- Dixon JB, Dixon AF, O’Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese: homeostatic model assessment. Diabet Med 2003; 20:127–134.
- Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res 2004; 36:735–741.
- Wajchenberg B, Cohen R, Schiavon C, et al. Laparoscopic duodenal jejunal bypass in the treatment of type 2 diabetes mellitus in patients with BMI < 34. Initial results. Abstract presented at: 68th Annual Scientific Sessions of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 1454-P.
- Schauer P, Chand B, Brethauer S. New applications for endoscopy: the emerging field of endoluminal and transgastric bariatric surgery. Surg Endosc 2007; 21:347–356.
- Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244:741–749.
- Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 2008; 68:51–58.
- Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc 2004; 60:114–117.
- Rattner D, Kalloo A, SAGES/ASGE Working Group on Natural Orifice Translumenal Endoscopic Surgery. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. Surg Endosc 2006; 20:329–333.
How should we introduce and evaluate new procedures?
By Joel D. Cooper, MD
Time magazine published an article in 1995 titled “Are Surgeons Too Creative?” that examined the question of whether operations should be regulated the way that medications are.1 The piece featured two patients. One, a patient with emphysema who underwent lung volume reduction surgery at our institution during the early days of this procedure, had a good outcome. The other was a neurosurgical patient who had a bad outcome.
The public is somewhat sympathetic to this article’s premise, which can be viewed as a call to require a similar level of evidence for surgical procedures as for new drugs. This sympathy arises from the expense of new technologies, pressure from payors to control costs and increase profits, hospital budget restraints, and the reality of increasingly well-informed patients.
Yet there are distinct differences between drugs and surgery. A new drug does not change over time. A new drug is associated with a variable biologic response whose assessment often requires large numbers of patients and considerable follow-up. And a new drug may manifest unforeseen late side effects and toxicities far removed from the time of initial use. In contrast, none of these characteristics applies to surgical procedures. A surgical intervention changes over time as the technique and experience evolve and as refinements are made in patient selection and in pre- and postoperative management. With this evolution comes a change in risk over time. Patient selection for surgery is as much an art as a science; each patient requires assessment of both the potential benefits and risks of the procedure, which argues against offering an operation by prescription. Moreover, with surgery, the facilities and the operator’s skill and experience levels vary from one center to another.
INTRODUCTION OF NEW PROCEDURES: COVERAGE VS VALIDATION
Introduction of a new surgical procedure depends on the nature of the procedure and the other interventions that may be available for the condition. In assessing how new procedures should be introduced, I believe we need to distinguish between coverage and validation. Coverage—ie, payment for the procedure—is an economic issue, whereas validation involves an ethical and scientific evaluation of the role of the procedure.
Coverage by an insurer should have at least theoretical justification and presumption of benefit. For instance, the rationale behind a heart transplant for a patient with a failing heart is obvious. Coverage generally requires preliminary evidence of efficacy, possibly in an animal model, although no animal models may exist for some conditions. Most important, a different standard for providing initial coverage should be applied if no alternative therapy exists for a condition that is severe, debilitating, and potentially life-threatening; if a new procedure treats a condition for which a standard therapy already exists, the standard for coverage must be higher. Finally, coverage in all cases should require ongoing reassessment of the procedure.
In contrast, validation is a scientific analysis of results over time, including long-term results, and can be accomplished by well-controlled case series, particularly if the magnitude of the benefit is both frequent and significant and especially if no alternative therapy exists. Randomized clinical trials are the gold standard for appropriate interventions but are not always applicable.
A 1996 study by Majeed et al2 provides a good example of validation-oriented surgical research. In this blinded trial, 200 patients scheduled for cholecystectomy were randomized to either laparoscopic or open (small-incision) procedures. The study found no differences between the groups in terms of hospital stay or postprocedure pain or recovery. In an accompanying commentary,3Lancet editor Richard Horton praised the design and conduct of the study, noting that it was very much the exception in surgical research, which he argued was preoccupied with case series. Horton offered the following speculation about this preoccupation:
Perhaps many surgeons do not see randomised trials as feasible strategy to resolve questions about surgical management. Cynics might even claim that the personal attributes that go to make a successful surgeon differ from those needed for collaborative multicentre research.3
IS THE ‘SURGICAL SCIENTIST’ AN OXYMORON?
Barnaby Reeves, writing in The Lancet 3 years later, offered a more diplomatic take on the difficulty of evaluating surgical procedures:
What makes a surgical technique new is not always easy to define because surgical procedures generally evolve in small steps, which makes it difficult to decide when a procedure has changed sufficiently to justify formal evaluation.4
Reeves went on to argue that doing an evaluation too early may preclude acceptance, since the technique may not have evolved sufficiently and surgeons may not have mastered it; conversely, doing an evaluation too late may make the evaluation moot, since the technique may have already become established and withholding it may be deemed unethical. Additionally, he noted that the quality of surgical evaluation is complicated by the possibility that some surgeons have better mastery of—and therefore better outcomes with—one procedure while other surgeons have better mastery and outcomes with an alternative procedure.4
These concerns were well captured by the late Dr. Judah Folkman, whom I once heard say, “When a basic scientist is informed that another investigator cannot reproduce his work, it has a chilling effect; for the surgeon, however, it is a source of pride.”
RANDOMIZED TRIALS VS CASE SERIES: A TIME AND PLACE FOR EACH
Even as we recognize these challenges specific to surgical evaluation, we are still left with the task of determining when a randomized controlled trial is appropriate and when a case-control series may suffice.
There are three broad sets of circumstances in which a randomized trial is essential:
- For preventive procedures, ie, when the operation is done to reduce the potential for a future adverse event. An example would be evaluating carotid endarterectomy to reduce the potential for stroke in asymptomatic patients with 60% or greater stenosis. Only a randomized trial could have shown a difference in favor of endarterectomy over aspirin plus best medical therapy.
- To compare a procedure with alternative medical or surgical interventions. I would argue that laparoscopic surgery should have been introduced with randomized trials, as it begs one to suspend judgment and accept that small incisions are invariably and de facto better than a large incision.
- For trials in oncology, where the outcome depends on long-term results, such as survival or time to recurrence. Examples would include comparisons of surgery alone versus surgery plus chemotherapy for prevention of cancer recurrence.
Similarly, there are several scenarios in which a case-control series is appropriate and adequate:
- When no alternative therapy exists. Falling into this category, in my view, are lung transplant, which we introduced successfully at the University of Toronto in 1983, and lung volume reduction surgery, which we introduced in 1993.
- When the natural history of the condition is well documented and the impact of the intervention is obvious.
- When the magnitude of the procedure’s effect is measurable, significant, and expected.
RANDOMIZED TRIALS IN SURGERY
Advantages of randomized trials
Randomized clinical trials confer a number of advantages. They eliminate bias. They ensure a balance between treatment groups in terms of known or unknown prognostic factors. And, importantly, they have a major impact on payors.
A tale of two Medicare payment decisions
The impact of clinical trials on payors is exemplified by the contrasting stories of two procedures: transmyocardial laser revascularization and lung volume reduction surgery.
Transmyocardial laser revascularization (TMR) involves the creation of channels in the myocardium with a laser to relieve angina. Although TMR is a dubious intervention with no physiologic rationale (similar to internal mammary artery ligation for angina5) and no proven improvement in life expectancy (only a reduction in pain), it was approved for reimbursement by Medicare because it was investigated in a randomized trial.6 However, the “randomized trial” was not truly a randomized investigation because the control patients received only medical therapy and did not go to the operating room to receive a sham operation.6 Despite this flaw, the perceived authority of the trial was sufficient to influence Medicare.
In contrast, Medicare refused to pay for lung volume reduction surgery until it was subjected to a randomized trial, despite the fact that the procedure had produced tremendous benefit in hundreds of patients at multiple centers who otherwise could not have achieved such benefit. Only after $50 million was spent on a randomized controlled trial, the National Emphysema Treatment Trial (NETT),7–9 did Medicare agree to pay for lung volume reduction surgery. The trial showed that over 5 years, the procedure was associated with significant improvements in life expectancy, exercise tolerance, and quality of life, but the study took 8 years to conduct and by then it was a bit too late, as detailed in the following section.
NETT: A case study in how a trial can be counterproductive
Lung volume reduction surgery is an operation based on the recognition that the crippling effects of emphysema are hyperinflation of the chest, flattening of the diaphragm, and inability to move air in and out of the chest. The notion that the chest can be reconfigured in the patient with emphysema by removing the distending overinflated emphysema led us to develop the volume reduction operation.
The NETT was initiated by Medicare, and the protocol denied compassionate crossover of patients.7 In an attempt to establish clinical equipoise, surgeons who participated were not allowed to perform any volume reduction operations on non-Medicare patients or on Medicare patients not enrolled in the trial. After 2 years of slow patient enrollment, the clinical trial committee, in an effort to increase enrollment, eliminated the original entrance criteria specifying certain degrees of hyperinflation and diffusing capacity. An excess of mortality was discovered 2 years later in a subgroup randomized to volume reduction surgery;8 not surprisingly, further analysis showed that the excess mortality was largely confined to patients who would have been excluded based on the original entrance criteria. This is a matter of public record but was never acknowledged in published reports of the trial. Final 5-year NETT results showed that in patients with upper lobe emphysema, lung volume reduction surgery improved survival, increased exercise capacity, and improved quality of life.9 By the trial’s completion, however, the procedure’s reputation had been tarnished irreparably by bad publicity from the deaths attributable to the misguided changes to the original eligibility criteria.
Disadvantages of randomized trials
The NETT exemplifies many of the drawbacks of randomized trials in surgery, particularly the need to wait long periods while they are being conducted. During the 8 years in which the NETT was ongoing, the number of lung volume reduction operations declined, with the typical center performing fewer than 6 cases per year, on average. That limitation is certainly not conducive to the development of a new procedure for a disabling condition in patients with no ready alternative.
Other disadvantages of randomized trials in surgery are their considerable expense and the fact that they often are not generalizable and often are not appropriate. Moreover, when they are flawed, randomized trials propagate, sometimes for decades, misleading information that is nonetheless considered “authoritative.” For instance, lung cancer kills more men and women in the United States than the next three cancers combined, yet, on the basis of a flawed randomized trial,10 the American Cancer Society advises smokers to wait for symptoms before undergoing chest radiography, instead of recommending annual screening chest radiography. This is a major reason why two-thirds of lung cancer cases are discovered too late to save the patient.
‘Better to know nothing than to know what ain’t so’
Indeed, this potential for randomized clinical trials, when flawed, to propagate misleading information makes the perceived authoritativeness of randomized trials both an advantage and a disadvantage. As Berger and colleagues noted a few years ago, overuse of randomized trials for evaluating emerging operations could have led to the demise of heart transplantation, mechanical circulatory assist devices, cardiac valve procedures, coronary bypass grafting, and repair of congenital lesions.11
For this reason, one of our responsibilities when reading the literature and conducting studies is not just to answer unanswered questions but to question unquestioned answers. As 19th-century humorist Josh Billings put it, “It’s better to know nothing than to know what ain’t so.”
A PERSONAL PERSPECTIVE
In my view, health care providers should restrict the application of new procedures to a limited number of centers of excellence that have appropriate resources and experience. Those centers should be required to document and report specified information regarding morbidity, mortality, and objective measures of outcome; if they do not comply, they should lose the privilege of doing such research. The data should be reviewed by an independent, nongovernmental scientific panel. In this way, the procedure can be offered to appropriate patients, insurers and patients can be protected against abuse, and the necessary data can be collected for objective analysis.
Idea to implementation: A personal perspective on the development of laparoscopic nephrectomy
By Ralph V. Clayman, MD
Change in the surgical world involves three aspects, which I refer to as the three Ds: discovery, development, and dissemination. Change requires proof that the new method is superior to the old. When we, as innovators, develop something “new,” I believe that our immediate subsequent task is to do everything we can to prove that this “new” finding is of no value whatsoever before we determine that it is worth advancing.
Getting to Malcolm Gladwell’s tipping point—the act or event after which nothing is ever the same— requires a team of people, usually from different disciplines, coming together to concentrate on a problem, or an individual whose experiences in different fields provides the ability to “see” the next level. In my opinion, one person working in one discipline rarely leads to breakthrough progress in medicine.
These observations about surgical innovation stem largely from my experience in the development of laparoscopic nephrectomy, in which I was privileged to play a role while at Washington University in St. Louis, which I will outline here.
THE HISTORY BEHIND LAPAROSCOPIC NEPHRECTOMY
After doing preliminary work in dogs, the German surgeon Gustav Simon performed the first human nephrectomy in 1869, in a woman with a ureteral vaginal fistula. The operation was a success: it took him 50 minutes to complete the procedure, and 6 months later the patient went home.
From that point in 1869 until 1990, progress in nephrectomy was minimal, with open surgery remaining the gold standard. While the surgeon’s tools remained largely unchanged, the advances that did occur were in anesthesia, analgesia, and antibiotics, which allowed patients to better survive the onslaught of the operation.
In an unrelated arena, laparoscopy was developed in 1901 by another German surgeon, Georg Kelling, who pumped air into the peritoneal cavity of a dog in a successful effort to stop bleeding from the stomach. Within the pneumoperitoneum, Kelling was able to examine the canine organs with a cystoscope at pressures as high as 140 mm Hg. This discovery was not applied clinically, however, until 9 years later when the Swedish gastroenterologist H.C. Jacobeus used Kelling’s pneumoperitoneum concept and a cystoscope to visualize the peritoneal cavity to search for cancer. The technique advanced little in the subsequent decades —apart from Semm’s seminal laparoscopic removal of the appendix in the 1960s—until 1985, when the first laparoscopic gallbladder removal ignited the era of laparoscopic cholecystectomy.
Three technological developments spurred this recent surge in laparoscopy: (1) the ability to affix a camera to the endoscope, (2) the ability to display the camera’s images on a video screen, and (3) the development of self-feeding clip appliers to allow occlusion of vascular or ductal structures.
THE EVOLUTION OF LAPAROSCOPIC NEPHRECTOMY
Discovery
I became interested in the possibility of laparoscopic nephrectomy during the laparoscopic cholecystectomy craze in the late 1980s. At that time, I was working with Dr. Nat Soper, performing laparoscopic cholecystectomies in pigs to show that the procedure could be done safely with electrocautery rather than a laser. As it turns out, the anatomy of the porcine kidney is such that the colonic reflection lies medial rather than lateral to the organ. As such, the kidney is quite visible as soon as one enters the abdomen. Indeed, the kidney seemed to be saying to us, “Hey, what about me? I could come out through that hole too.” That is basically how the idea arose.
So, along with Dr. Lou Kavoussi and many others in our research team, we attempted laparoscopic nephrectomy in the pig and succeeded: the kidney could be removed through a small hole by entrapping it in a sack, breaking it up in the sack, and pulling it out.12 The team involved in this discovery were specialists in urology and general surgery as well as biomedical engineers from industry, specifically a team from Cook Urological led by Mr. Fred Roemer.
After performing this technique numerous times in the laboratory, we reduced the operation’s duration to 90 minutes, at which point we believed the procedure had advanced sufficiently to be considered for clinical use.
Development
The patient we selected for the initial clinical case was an 85-year-old woman with a 3-cm mass in her kidney. She was deemed to be “too sick to operate on,” so she was presented to me as a candidate for the new laparoscopic procedure.
Amazing as it may seem in our current medical climate, at that time (1990) we were faced with the question of whether or not to seek institutional review board (IRB) approval. The argument could be made that since radical nephrectomy had been practiced for 120 years and laparoscopy had been around for nearly 100 years, the combination of these two well-accepted procedures might require nothing more than physician-patient informed consent. However, the concept of “informed consent” in this context was problematic: what could we tell the patient about a procedure that had never been done before except that if it was not working out we would convert to the standard open procedure?
A senior colleague—actually my boss at that time, Dr. Bill Catalona—sagely advised me to get IRB approval, noting, “If the operation works out well, you’ll be fine, but if it doesn’t work out well, they’ll kill you if you don’t have approval.” So we fortunately ended up seeking (and receiving) IRB approval, as well as providing, as best we could, informed consent to the patient and her best friend.
Our next consideration was designating a team member to determine if and when conversion to open surgery would be necessary. We needed a “referee” to aid in objectively determining a point at which we should convert. For our team, that person was Dr. Teri Monk, our anesthesiologist, who had no previous experience with our laboratory work but understood what we were attempting.
So we proceeded with the first clinical laparoscopic nephrectomy on June 25, 1990. The kidney was embolized the morning of the procedure. Five laparoscopic ports were placed. The clip appliers proved too small for renal vein occlusion, so the main renal vein had to be traced to its branches; in total we clipped five separate sets of renal vascular branches. The kidney was ensnared and morcellated, which took 7 minutes. Total operative time was 6.8 hours. The complications that arose were not anticipated:
- Intraoperative oliguria due to the prolonged pneumoperitoneum
- Fluid overload (postoperative congestive heart failure) due to providing fluids to the patient as though this were an open procedure
- Dilutional anemia, again due to providing excessive fluids for a closed procedure.
Postoperative pain medications consisted of one dose of morphine sulfate. The patient was discharged on postoperative day 6 and resumed normal activities by postoperative day 10.13
Dissemination
Before a new procedure is disseminated, evidence of the four Es—efficiency, effectiveness, equanimity, and economy—must be obtained. In retrospective reviews, laparoscopic removal was associated with a slightly longer operating time but much less blood loss, a shorter hospital stay, and fewer complications. The immediate cancer cure rate was the same for open and laparoscopic nephrectomy, and over time the laparoscopic procedure has been shown to be just as good as open surgery at 5 and now 10 years. Also, with time, laparoscopic nephrectomy was shown to reduce institutional costs.
The next question was the proper way to disseminate this knowledge. At Washington University we took the traditional route of providing courses, offering 17 courses on laparoscopic surgery to nearly 1,000 urologists from 1985 to 2002. But as Winfield and associates later showed, only 54% of urologists who completed a 2.5-day hands-on, laboratory-based laparoscopic course actually ended up introducing laparoscopy into their practice.14
The challenge of dissemination is still with us, and we need to find better methods of transferring new skills to our surgical colleagues. In this regard, longer experiences, such as weeklong mini-fellowships and the development of procedure-specific surgical simulators, hold great promise.
UNANSWERED CHALLENGES, UNMET NEEDS
With the advent of any technology comes a cornucopia of unanswered questions and challenges. In the areas of discovery and development, a key question is whether every procedure performed using a new Food and Drug Administration (FDA)-approved technology requires a separate approval by the IRB and ethics committee. For instance, if robotic prostatectomy is approved and performed, are separate approvals needed for robotic nephrectomy, robotic pyeloplasty, and robotic vasectomy? Where would or should the approvals end?
With respect to dissemination, many questions remain: How is a new technology taught effectively? How is surgical competency tested? How is clinical performance or proficiency evaluated?
One problem specific to dissemination is a lack of funding. While ample funding is available for discovery and development, as they bring prestige and profit, dollars are scarce for dissemination, or the teaching and testing of competency and proficiency with new procedures.
Evidence of our failure to educate the postgraduate surgeon abounds in terms of poor outcomes and malpractice suits. The response of government all too often is the knee-jerk reaction to protect (ie, regulate), not educate. To be sure, we can do better, but only if our society commits to the process—not with words, but with funded educational action.
With regard to the last, I believe there is an unmet need for the development of accurate, validated surgical simulators. As a society, we need to find a way to fund the development of simulators for each surgical subspecialty and then use these devices to objectively test an individual surgeon’s manipulative skill as well as cognitive ability when he or she seeks certification or recertification—and perhaps, albeit in an abbreviated 5-minute format, before beginning each operative day. We owe this to ourselves, but most of all to our patients, who in all confidence place their lives in our hands.
Special perspectives in infants and children
By Thomas M. Krummel, MD
If we surgeons take a step back and consider for a moment what has changed in the operating room (OR) in the past 50 to 60 years, the clear answer is, “Just about everything.” The monitors, pumps, transport devices, and OR tables and lights have all changed dramatically, as have the tools, catheters, sutures, energy sources, scopes, staplers, ports, valves, and joints. If we consider technologies outside the OR that guide what we do inside the OR, the changes are just as striking. Circulatory assist devices for the failing heart and widespread use of dialysis for the failing kidney postdate 1950, as does all of our modern imaging capability—ultrasonography, computed tomography, magnetic resonance imaging, positron emission tomography, functional imaging. As for pharmacotherapy in 1950, there were three antibiotics, no antivirals, one antifungal, and three chemotherapeutic agents. Open drop ether was the anesthetic of choice. Not only have the tools and technologies changed, but virtually every procedure has been changed. Both our profession and the industry that has developed these devices and tools can be rightfully proud.
It is likewise necessary to recognize that our patients have been partners in this innovation. Many of them have given informed consent to participate in research and experimental procedures with the expectation that the benefits might accrue only to future patients and not to themselves. That is a hell of a contribution, and we can be proud of our patients’ partnership.
THE GOOD, THE BAD, AND THE UGLY OF INNOVATION
The history of progress in surgical care is always about innovation, and such progress almost always begins with an unsolved patient problem, regardless of the solution that is developed, be it a tool, a device, a technology, or a surgical procedure. At the same time, any discussion of the ethics of surgical innovation should recognize that while efforts to solve patient problems over the years have had many good results, they have also had some bad results and even the occasional ugly result.
This conference has already focused on much of the good that has come from surgical innovation, including transplantation, remarkable advances in cardiac and gastrointestinal surgery, a host of devices, and too many other benefits to list. Yet missteps have been made along the way, such as bloodletting, gastric freezing as a therapy for ulcer, and carotid denervation for treatment of asthma in children.
Then there are the ugly incidents, and these notably include a number of cases involving children, an issue of special interest to me as a pediatric surgeon. Consider the following examples:
- Edward Jenner’s notorious cowpox experiment in the late 18th century was conducted in an 8-year-old boy.
- A well-documented literature shows that orphans were used as subjects for tuberculosis and syphilis inoculations.
- The more recent case of Jesse Gelsinger involved a teenager with a nonlethal condition who died in a clinical trial of gene therapy, after which an undisclosed financial interest on the part of one of the treating physicians was revealed.
It should give us pause to note that many of these practices that look foolish in hindsight probably seemed more rational at the time they were undertaken.
CHILDREN: THE ORPHANS OF INNOVATION
Children have been the orphans of innovation, as technology development specifically for children has traditionally been a low priority. There are several reasons for this:
- FDA standards for approving therapies in children are high. For instance, the vast majority of chemotherapeutic drugs are not approved for use in children because conducting a trial specifically in children is deemed too expensive.
- Pediatric markets for therapies are small.
- The payor mix is poor.
The benefts of duality
Nevertheless, children have benefited enormously from the duality of technology development, in which a technology developed for one population—either adult or pediatric—ends up benefiting both populations. For instance, no one would have invented the pulse oximeter to care for a child, yet now it is the only device with which infants and children are monitored in the operating room and during transport.
Likewise, in some cases the solutions to pediatric problems have had reciprocal benefits in adults. Ligation of the patent ductus arteriosus and the Blalock-Taussig shunt for tetralogy of Fallot opened the door to our understanding of surgery on the great vessels and ultimately enabled the development of cardiac surgery. Similarly, the early impetus for Thomas Starzl’s groundbreaking work in transplantation was focused on children with biliary atresia even though this work is now much more widely applied in adults.
ETHICAL PRINCIPLES APPLY EQUALLY TO ADULTS AND CHILDREN
The principles of medical ethics that began with Hammurabi in 1750 BC and progressed through Hippocrates’ work circa 400 BC, the 1946 Nuremberg medical trial, the 1964 Declaration of Helsinki,15 Henry Beecher’s classic exposé in 1966,16 and the 1979 Belmont Report17 are just as valid for children as they are for adults.
Francis Moore, the great surgeon who created the environment and the team at Brigham and Women’s Hospital that facilitated the first twin-twin transplant, identified six important components of ethical surgical innovation:18,19
- A solid scientific background (basic laboratory research)
- A skilled and experienced team (“field strength,” as Moore called it)
- An ethical climate within the institution
- An open display for ongoing discussion
- Public evaluation
- Public and professional discussion.
The principles behind these components remain as true today as they were 20 years ago when Moore outlined them.
SPECIAL CONSIDERATIONS IN PEDIATRIC SURGERY: A CASE STUDY IN MATERNAL-FETAL MEDICINE
The Belmont Report, mentioned above, was developed by the US government in 1979 to form the basis of regulations for federally funded research involving human subjects.17 The report identified three basic principles that must underlie such research:
- Respect for persons—protecting the autonomy of all subjects, treating them with courtesy, and allowing for informed consent
- Beneficence—maximizing benefits from the research initiative while minimizing risks to the subjects
- Justice—ensuring reasonable, nonexploitative, and well-considered procedures that are administered fairly.
In pediatric surgery, everyone agrees that the “best interests of the child” must be protected, but the issue of autonomy (a key element of the first Belmont principle) is more difficult to define, of course, when the patient is a child rather than an adult. The question of autonomy is especially tricky in the evolving field of maternal-fetal medicine: what if the patient is a fetus and the mother is an innocent bystander?
Over the past 20 years, tremendous progress has been made in our understanding of diseases of the fetus, particularly diseases that limit fetal viability and diseases that cause serious organ damage but which may be more responsive to postnatal therapy if they are treated prenatally. Michael Harrison, N. Scott Adzick, and a few of their disciples have laid the ethical groundwork for consideration of the fetus as a patient.
Considerations in maternal-fetal medicine
I will conclude with a case in maternal-fetal medicine for us to consider and perhaps debate in the panel discussion at the end of this session. As you consider this case, keep in mind several important observations relating to maternal-fetal medicine:
- The mother’s health interests cannot be underestimated.
- Most “fixable” fetal lesions (ie, those that interfere with development and cannot be fixed postnatally, but for which intervention in utero may result in normal development) are very rare. They include obstructive uropathy, lung lesions causing hydrops, congenital diaphragmatic hernia, sacrococcygeal teratoma, hydrocephalus, twin-twin transfusion syndrome, congenital high airway obstruction, hydrothorax, myelomeningocele, and congenital heart disease.
- The field is evolving, and the efficacy of therapy is supported by variable level I, II, and III evidence.
- The law has not kept (and perhaps cannot keep) pace with developments in this field.
Case study
A 24-year-old healthy woman has a fetus of 28 weeks’ gestational age with progressive lower urinary tract obstruction with megacystis, bilateral hydronephrosis, and oligohydramnios. In other words, there is diminished volume in the uterine cavity that causes compression of the fetal chest and subsequent respiratory compromise that will be fatal if not addressed. The karyotype is a normal 46,XY male. Serial urine sampling reveals electrolyte and protein profiles with a good prognosis.
Prenatal counseling with fetal therapy specialists suggests that this is the “perfect case” for a vesicoamniotic shunt. This is the least invasive, most successful fetal surgical intervention. It is done under local anesthesia and involves transabdominal transuterine percutaneous placement of a double-lumen pigtail catheter in the fetal bladder. There has never been a reported maternal death, and morbidities have been minimal. Renal and pulmonary function both are improved by approximately 80% in fetuses treated with this intervention, and survival is improved.
The father is eager to proceed. The mother is ambivalent. Should the mother be pressured to proceed, for the good of the child?
Questions to ponder
The following questions are intended to be provocative, with no clear-cut answers:
- Should (or does) the fetus have independent moral status? Is it full, graded, or none? Does it matter?
- What are the beneficence-based obligations to the fetus? At 28 weeks’ gestation, the fetus is viable outside the uterus. The fetus is otherwise well, without a lethal karyotype, and has currently good renal function.
- What are the beneficence-based and autonomy-based obligations to the mother? What are the mother’s obligations to the fetus?
- What if the mother ultimately decides to proceed and the insurance company denies coverage? What are the social responsibilities to care, cost, and research?
These questions lend themselves to discussion. As much as we surgeons like to be certain about what we do, we would do well to heed the quote from Voltaire that the great surgeon Norman Shumway hung on his office door: “Doubt is not a very agreeable state, but certainty is a ridiculous one.”
Bariatric surgery: What role for ethics as established procedures approach new frontiers?
By Philip R. Schauer, MD
Obesity is a staggering problem: 100 million Americans are overweight, 85 million more are obese, and another 15 million are morbidly obese (ie, ≥ 100 lbs above ideal body weight). The incidence of obesity is rising rapidly and threatens to shorten the life spans of today’s young generations relative to their parents. Unlike other conditions, such as cardiovascular disease and cancer, obesity has seen no widespread progress in management in recent years.
Recognition of obesity as a medical problem is a challenge in itself. Many people consider obesity to be a character flaw or a behavioral issue and fail to recognize it as a disease entity. Yet obesity is the root cause of many metabolic conditions and diseases with metabolic components, including type 2 diabetes, heart disease, blood pressure, metabolic syndrome, acid reflux, gout, arthritis, and sleep apnea.
The approach to obesity treatment can be conceptualized as a pyramid, with the aggressiveness of the intervention based on the patient’s body mass index (BMI). At the base of the pyramid, for patients with lower BMIs, are minimally invasive (and minimally effective) interventions involving changes in diet, physical activity, and other lifestyle factors. As BMI increases, so does the intensity of treatment, to include pharmacotherapy and eventually bariatric surgery. Traditionally, surgery has been considered only at the very top of the pyramid, for morbidly obese patients, and is usually not offered as an option for the vast majority of people with this condition.
The sad reality is that the various combinations of these therapies are effective in fewer than 1% of the approximately 100 million Americans who are obese. Because surgery has been shown to be the most effective therapy for obesity, the remainder of my discussion will focus on surgery, with an eye toward potential new indications for bariatric procedures and the questions they raise.
SURGICAL APPROACHES TO OBESITY
Bariatric surgery has evolved over the past 50 years. Although there are about a dozen different permutations of bariatric procedures performed in the United States today, they fall into one of three major types of operations, as outlined below:
Gastric banding reduces appetite and satiety by adjusting and tightening the gastric band. This procedure has been in existence for 10 to 15 years and represents about 25% of operations for obesity in the United States.
The biliopancreatic diversion procedure diverts most of the small bowel and radically reduces absorption of calories. Patients undergoing this procedure lose weight because few calories are absorbed into the body. This approach, while quite effective, is somewhat radical and represents only about 2% of the operations for obesity in the United States.
The Roux-en-Y gastric bypass procedure has been the dominant procedure over the past 15 to 20 years. A combination of the above two procedures, it involves reducing the gastric reservoir and bypassing the stomach and upper intestine. The reduction in gastric volume reduces calorie intake by enhancing satiety, and the limited foregut bypass moderately reduces absorption.
No randomized trials, but much support from observational studies
Virtually none of these procedures evolved with randomized controlled trals. Instead, they evolved incrementally, primarily on the basis of knowledge gained from case procedures. Despite the lack of randomized trials, these operations have been shown to be effective, particularly in patients with multiple metabolic abnormalities associated with severe obesity. A large body of data from case-control and cohort studies demonstrates not only dramatic improvement in metabolic abnormalities with the use of various bariatric procedures, but also improvements in quality of life and survival.20–26 The two most recent of these studies, published in 2007, found reductions in mortality of 29% (adjusted) and 40% among surgical patients compared with well-matched obese controls during mean follow-up of more than 7 years.25,26 Reductions in the incidence of cardiovascular mortality and, secondly, cancer-related mortality were the two major contributors to the overall mortality reduction in these two studies. Consistent with this latter finding, obesity is starting to be thought of as a disease that may lead to cancer.
NEW FRONTIERS FOR BARIATRIC PROCEDURES
The current indications for bariatric surgery have existed intact for about 25 years, and were based on limited evidence available at the time. They are basically as follows, assuming acceptable operative risk and appropriate patient expectations:
- BMI greater than 40 kg/m2
- BMI greater than 35 kg/m2 with significant obesity-related comorbidities.
Payors adhere strictly to these indications, such that they will not pay for bariatric surgery in a patient with a BMI less than 35 kg/m2. This raises questions about the appropriateness of such a firm threshold and whether expansion of these strict indications may be reasonable.
Even without broadened indications, the volume of bariatric procedures in the United States has grown dramatically in recent years. Whereas only 10,000 to 20,000 of these operations were performed annually in the 1990s, approximately 200,000 such procedures were performed in 2007, and this number is expected to double over the next 5 years or so.
This growth in volume has been paralleled by burgeoning media interest in bariatric procedures, particularly in the last few years. More attention can be expected as we increasingly recognize the potential of bariatric procedures for indications beyond strictly the treatment of morbid obesity. At least two new frontiers loom: metabolic surgery and endoscopic surgery.
Metabolic surgery
Procedures that incorporate a bypass—the Roux-en-Y gastric bypass and the biliopancreatic diversion —have been associated with a reversal of metabolic diseases such as type 2 diabetes.27–32 Many patients with type 2 diabetes who have undergone these procedures have been able to be weaned off insulin and insulin-sensitizing medications while maintaining normal blood glucose levels. The effect has been profound and immediate, occurring even before the patient loses weight. In one series of patients with type 2 diabetes who had undergone a bypass operation, 30% left the hospital in a euglycemic state.29
These observations have been made primarily in the morbidly obese population, who are the primary candidates for bariatric bypass procedures. However, because of the rapid improvement in metabolic abnormalities that has been observed, interest has arisen in applying these procedures to populations that are not morbidly obese. Bypassing of the foregut appears to be critical, perhaps because it tempers the release of hormonally active peptides from the gastrointestinal tract.33 In any case, the gut is regaining recognition as a major metabolic organ.
In light of these hypotheses, the duodenal-jejunal bypass is a bariatric procedure that may be beneficial for a patient with type 2 diabetes who is not morbidly obese. In this operation, the stomach volume is preserved but the foregut is bypassed. In a small experimental series from Brazil, patients with type 2 diabetes who were normal weight or only slightly overweight had resolution of their diabetes following this procedure, without any weight loss.34
New applications for endoscopy
Another area of development is endoluminal and transgastric bariatric surgery. Endoluminal surgery is performed entirely within the lumen of the gastrointestinal tract using flexible endoscopy. Transgastric surgery is performed within the peritoneal cavity, which is accessed via a hollow viscus. Both approaches use natural orifices to gain surgical access, thereby avoiding access incisions and scars.35
The benefits of such an approach are numerous:
(1) fewer complications and side effects; (2) less invasiveness, and thus the ability to perform in the outpatient setting; (3) reduced procedure costs; and (4) better access to treatment. The implication in terms of indications is the potential to use such procedures to prevent progression to morbid obesity.
Examples of these procedures are proliferating:
Gastrojejunostomy reduction is an endoscopic procedure that involves reducing the dilated opening of the gastric pouch after gastric bypass surgery. New endoscopic suturing or stapling devices enable the outlet reduction without requiring surgery. The result is enhancement of weight loss without a major operation.
Endoluminal suturing uses endoscopic instruments to suture the stomach to reduce its volume. When this procedure is perfected, the patient should be able to leave the endoscopy suite and return home within a few hours.
The duodenal sleeve is an avant garde concept in which an internal sleeve is threaded into the stomach and down the intestines.36 The sleeve covers the absorptive surface of the small bowel, preventing absorption of nutrients to cause weight loss. This procedure has been shown to have a strong antidiabetic effect as well.
Clinical applications of these operations are emerging. An endoluminal sutured gastroplasty procedure to shrink stomach volume has been shown in a small clinical trial to cause loss of significant excess body weight; the operation leaves no scars and is associated with a low risk of bleeding or any type of surgical complication.37 A similar procedure is in development that involves staples instead of sutures.
How best to validate innovations moving forward?
As we move into these new eras of metabolic surgery and endoluminal and transgastric bariatric surgery, interesting questions arise. We as innovators and caregivers are ethically obligated to demonstrate reasonable safety and efficacy before such new procedures are performed widely. Although some of these emerging procedures involve new devices that will go through the FDA review process, many are existing procedures for which indications may be expanded, while others are permutations of existing procedures for which no formal rules for validation exist. For new procedures that differ substantially from existing proven procedures but which do not require new devices, should we not be ethically bound to demonstrate safety and efficacy even though they do not require FDA review? These are the challenges that await as innovation takes bariatric surgery to new frontiers.
Natural orifice transluminal endoscopic surgery: Too much too soon?
By Christopher Thompson, MD, MHES
Although the endoscope has changed very little since the first fiberscope was developed 50 years ago, the accessories and other instruments used in conjunction with the endoscope have changed remarkably. These include clips for hemostasis, ultrasonographic technology, and instruments for tissue dissection.
These advances in endoscopy, combined with advances in laparoscopic surgery, have led to the convergence of these two fields, culminating in the new field of natural orifice transluminal endoscopic surgery (NOTES). In NOTES, the surgeon enters a natural orifice and punctures through a viscus to perform surgery, removes the endoscope, and closes the area without leaving a scar.
HISTORY OF NOTES AT A GLANCE
NOTES was patented as a concept in 1992. Its first application was as an exploratory procedure in the pig in 2004.38 Soon thereafter, therapeutic NOTES procedures in animals were reported, including tubal ligation, organ resection, cholecystectomy, and splenectomy.
Particularly notable in the development of NOTES is the extremely short interval between early animal experiments (2004) and the first human procedures, which took place as early as 2005 when surgeons in India used the technique to perform a human appendectomy. Since then, more than 300 NOTES procedures have been performed in humans throughout the United States, Europe, Latin America, and Asia, for applications ranging from percutaneous endoscopic gastrostomy rescue to transvaginal cholecystectomy.
This rapid adoption of NOTES in humans is concerning, as it raises clear questions about whether there has been time for adequate oversight and safety assessment. For instance, at a surgical conference in April 2008, questions and debate swirled around whether a large Brazilian registry of more than 200 NOTES cases did or did not include two deaths. Other ethical issues raised by NOTES are discussed further below.
DRIVING FORCES BEHIND NOTES
The medical rationale
Abdominal wounds can cause pain, are unaesthetic, and are prone to wound infections, ruptures, and hernias. They sometimes cause adhesions or may lead to abdominal wall syndromes with scar neuromas that cause pain later. They also require general anesthesia. Beyond these shortcomings of incision-based procedures, NOTES offers potential reductions in length of stay and therefore in cost. Moreover, certain patient populations may specifically stand to benefit from NOTES, such as obese patients, those with abdominal mesh in place, and those undergoing palliative procedures. This is the essence of the medical rationale for NOTES, which is somewhat thin.
Professional organizations and courses
In July 2005, leaders from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons convened a working group to support and plan for the responsible development of NOTES.39 The group formed the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR), an organization that has since sponsored several conferences on NOTES and procured millions of dollars in grants for NOTES research in animals. (In the interest of full disclosure, I am one of the founding members of NOSCAR.)
Additionally, leading institutions in this field have held numerous hands-on courses on NOTES throughout the United States, Europe, Latin America, and Asia. These courses, including those held by my laboratory at Harvard University, are designed to teach colleagues at other institutions how to set up an appropriate animal laboratory and to promote and encourage proper research in NOTES. There have been unintended consequences, however, as we have learned that some course attendees have returned to their home countries and immediately started using the techniques in humans.
New technology
At the July 2005 working group meeting that launched NOSCAR, we determined that several technological advances were needed before NOTES could be safely applied to humans. These included development of multitasking platforms, better devices for tissue apposition and fixation, better imaging and spatial orientation, and improved means of retraction.39 Industry responded with novel devices and end effectors such as guide tubes, direct drive systems, endoscopic suturing devices, magnetic retraction, devices for closing luminal defects, flexible staplers, and computerized robotics.
Other driving forces
Additional forces have undoubtedly contributed to the rapid development of NOTES:
- The slowdown in innovation in general surgery in recent years has left a vacuum to be filled.
- An abundance of venture capital has been available to rush into that vacuum.
- Perceived patient demand (owing to cosmetic advantages) has been a driver, especially in cities such as Rio de Janeiro, Milan, and New York.
- The fear of being left behind is a factor that cannot be underestimated. Surgeons who failed to convert to laparoscopic techniques from open techniques in the early 1990s for procedures such as cholecystectomy, fundoplication, and splenectomy were losing their patient bases. Many surgeons fear a similar phenomenon today if they do not adopt NOTES into their practices.
ETHICAL ISSUES RAISED BY NOTES
As NOTES moves toward further evaluation in humans, several ethical questions need to be grappled with:
- Must there be a significant potential for improvement in care before an innovation advances to human research?
- Is the cosmetic benefit of NOTES sufficient, considering the substantially increased risk? For instance, laparoscopic cholecystectomy is well established, whereas NOTES cholecystectomy carries an increased risk of bile duct injuries and other injuries. Is NOTES worth the risk?
- What about the corporate agenda behind new technologies and its associated influence on the media?
- Are hospital IRBs adequate to the task of evaluating and monitoring these questions, and will they be independent of the impact of hospitals’ larger agendas?
Finally, the problem of premature adoption of this technology is particularly concerning. I heard a surgeon explain at a course that he performed NOTES on a few pigs at a previous course and then returned home to Peru and immediately started performing it on patients at his ambulatory surgery center. There is also the temptation for well-respected surgeons to go to other countries to practice their NOTES skills before returning to the United States, in hopes that their experience will help them attain IRB approval. Practices like these raise questions about what ethical responsibilities lie with those of us who have pioneered the technology and are trying to develop and disseminate it responsibly. We can try to vigilantly watch course attendees from certain countries, but there is little we can do in the absence of regulation and enforcement in those countries. These are difficult ethical challenges.
Panel discussion
Moderated by Jonathan D. Moreno, PhD
Dr. Jonathan Moreno: I would like to begin with any questions that the panelists have for one another.
Dr. Philip Schauer: I would be curious to hear how my colleagues define incremental changes in a procedure. In other words, what constitutes a new procedure versus a modification of an existing one?
In bariatric surgery we are grappling with a procedure called the sleeve gastrectomy, which poses challenges comparable to lung volume reduction surgery as described by Dr. Cooper. Many of us believe that this procedure is just a slight modification of a gastroplasty, yet payors consider it an entirely different procedure, and some want 5 to 10 years of follow-up data before they will pay for the operation.
Dr. Joel Cooper: That is not an easy question, but I would approach it from the standpoint of what you would tell the patient. When we were first developing lung volume reduction, we performed it only in patients who had absolutely no other alternative. Only later in its development did we offer it as an alternative to transplantation. How do you approach the patient when you can already achieve a very good result with an existing procedure and you can tell the patient, with some assurance, what to expect with that procedure? In the case of NOTES, I do not think that the cosmetics are sufficient justification.
The second aspect is regulatory. I am not a supporter of the FDA’s practices for the introduction of new procedures, but I believe strongly that universities have been derelict in setting the standard for the introduction of new procedures, particularly minimally invasive procedures. They have been using these procedures as marketing tools to vie with private hospitals for dollars and patients. I cannot say whether the rapid promulgation of these procedures at too early a stage actually can be prevented, but I do not recall the chairmen of major surgery departments getting together to issue public statements about the proper protocol for introducing new techniques. As Pogo said, “We have met the enemy and he is us.”
This may not answer your question, but I believe there should be no payment for any new or novel procedure for a certain period after its introduction, and certainly the hospitals should not be able to profit from it, although the expenses of a new procedure may be recouped. That alone would perhaps put the brake on some of the marketing and the financial incentives, and it might separate, to some degree, the development of new procedures from economic interests.
WHO SHOULD OBTAIN INFORMED CONSENT?
Dr. Moreno: Should informed consent be obtained only by a knowledgeable third party rather than the surgeon-innovator?
Dr. Thomas Krummel: The question is whether there is a disinterested third party who truly is knowledgeable; in cases where there is such a person, I see no downside to having that person involved. However, the notion of having someone who is not associated with clinicians or surgeons obtaining informed consent makes me uncomfortable. Informed consent is not a piece of paper. It is a trust between physician and patient, and to ignore that could leave you in a heap of trouble.
Dr. Cooper: I agree, but another process is important as well. In proposing lung transplantation before there had been any successful transplants, we defined in advance the standards, indications, and contraindications that we thought should apply. We did this in the absence of any particular patient, and it relieved us of the difficulty of making arbitrary decisions that may have led to unfairly accommodating one patient over another. Once the standards have been set in this way, they can be applied—whether by the investigator or by a committee—in an objective way to the group of patients that is most appropriate in the early phases of development.
Dr. Ralph Clayman: It is difficult for the inventors of an operation to dampen their enthusiasm for their creation to a point where they are as objective as they should be. Joel is bringing up situations for which there are no alternatives. My realm is an area in which there were well-established alternatives for everything we have done laparoscopically or percutaneously, and it was difficult to decide the indications or contraindications early on. Often, the early indications only had to clear the threshold of not seeming ridiculous.
The early development of percutaneous stone removal at the University of Minnesota took place entirely outside the purview of an IRB. Percutaneous nephroscopy had been around since 1955, and we extended it to plucking out a stone. That is how that entire field developed. Early on, we were not going to go after a stone that was as big as a fist because we did not have a way to break it up. As time went by, however, it evolved to the point where there was no stone in the kidney—regardless of its size, location, or hardness— that could not be removed through a small hole in the back. But that entire evolution proceeded without IRB approval.
For laparoscopic nephrectomy, for which there were well-known alternatives, who should have obtained the informed consent? Should it have been me, bringing along the “white coat” factor and not being able to really explain the potential problems since nobody had yet gone there, despite my rapport with the patient? Or should it have been a third party with whom I had discussed the procedure and its possible problems? I do not know the answer, but it raises an interesting point, especially in this age of IRBs and ethics committees.
Dr. Krummel: It is not unlike what we have tried at Stanford when we are not sure of the boundary for IRB consultation. The surgical chairs are willing to convene and essentially police one another, so that when the neurosurgeon proposes a brain transplant, there probably will be a pretty interesting conversation before it gets the green light.
Dr. Cooper: My experiences with IRB involvement differed quite a bit between my work in lung transplantation and my work in volume reduction surgery, but the differences owe a lot to the countries where I was practicing at the time. I did my early work in transplantation in Canada, where I did ask for approval from my hospital’s ethics committee and other relevant committees. In Canada, the hospital had a global budget, and it made a decision that it was willing to use part of its budget for transplantation. We received no fees for years, until the operation was proven to be effective, but that did not stop us from developing the procedure.
I had returned to the United States when I began my work with lung volume reduction, and I did not ask the IRB for permission to do that procedure. My justification was that, theoretically, volume reduction was similar to accepted practices for removal of nonfunctioning lung to improve respiratory mechanics (bullectomy) and that we would simply be applying the concept to a different group of patients. However, unlike in Canada, I did not have institutional financial support for doing this new procedure, so how was I going to do it if the hospital could not receive payment for it? I went to the IRB, but instead of asking for permission to do the procedure I asked for permission to study the procedure and to collect data on it. In that way, I was notifying the IRB of my action and thus giving it an opportunity to act. If I had gone to the IRB to approve the procedure, however, the operation would have been labeled experimental by insurance companies, who would have then found a way to deny payment. At least that was how it was in those days.
MARKETING OF MEDICINE: IS THERE NO TURNING BACK?
Question from audience: What makes you think that in 10 years there won’t be 100 million obese Americans watching television ads for noninvasive bariatric surgery promising to rid them of their obesity problem? What will keep that from happening?
Dr. Krummel: Nothing. What makes you think it is not happening now? Just look at the ads for the Lap-Band in the lay press.
Dr. Clayman: We already have direct marketing of drugs and direct marketing of facilities. What Joel said is true: “the enemy is us.” When I was in training, the idea that a physician would advertise was considered unethical. I still consider it thus. But everybody is doing it, so should that make it acceptable? I think not.
The same thing is true of the huge amount of money spent marketing drugs on television. Why should a single nickel be spent to advertise health care beyond generically informing the public of important health care issues and initiatives? You cannot go to an airport without seeing a surgical robotics program being advertised or a hospital being advertised. You cannot turn on National Public Radio without hearing well-financed spots touting the achievements of a hospital. You cannot watch television without seeing ads for erectile dysfunction medications or other new drugs. It is a waste of dollars. If we took all of that money and redirected it, we could probably solve much of the indigent health care problem, but we as a society have chosen not to do that.
SHOULD THE BAR BE RAISED FOR SURGICAL TRIALS?
Dr. Moreno: Let’s consider some additional questions. Why shouldn’t the government raise the bar on the level of evidence needed to gain regulatory approval for new devices? Why not require randomized trials, as is done for drugs?
Dr. Cooper: Procedures that lend themselves to a randomized trial should be studied at a limited number of centers with mandatory reporting and preset indications for promulgation and payment. I believe that universities have been derelict in their duty to require this level of evidence.
This question is always nuanced, however. Consider the case of laparoscopic procedures. They offer the advantage of smaller incisions, yet how many patients have had to die or suffer serious consequences for the sake of these smaller incisions? On the other hand, how many patients may have been saved from pulmonary embolism, wound infections, or a prolonged hospital stay as a result of laparoscopic techniques? Only a randomized trial could demonstrate whether or not there has been an overall payback from new procedures such as this, although even then the payback may be present for some types of patients but not others.
Dr. Schauer: The problem is expense. Perhaps it is all a matter of economics. Return on investment for the drug industry is something like 10 to 1, but return on investment for the medical device industry is generally much lower. Therefore, conducting large randomized controlled trials is extremely expensive and much more complicated for a device or procedure. This may explain why the standard for trials is different for the two industries.
Dr. Krummel: Virtually all fetal surgical procedures have been subjected to a trial, several of them randomized. The National Institutes of Health paid for many of these trials. One such study prevented rapid uptake of the congenital diaphragmatic hernia operation, which has never been proven in a randomized trial to be better than our current therapy. It is a good example of a randomized trial making a difference.
Dr. Clayman: As Joel pointed out, surgery is constantly evolving, whereas a drug remains unchanged throughout its lifespan. If we had started a prospective randomized trial after we had done our first laparoscopic nephrectomy, the procedure would have died because we were not nearly as facile with our first 10 as we were after our first 100. The technology continues to develop, and the surgeon continues to develop his or her skills, which makes a study of this nature overly dynamic. Perhaps the best you can do is a retrospective, matched, controlled study with the same surgeon, comparing his or her results after 40 or 50 laparoscopic procedures with results after his or her 50 most recent open procedures.
Dr. Cooper: How do you put a brake on the system? Would some sort of limited trial perhaps put a brake on the too-rapid promulgation that we often see?
Dr. Clayman: In the general surgery realm, laparoscopic cholecystectomy came out of private practice. It did not come out of the university with its faculty and laboratories dedicated to exploration and investigation. It never was properly vetted in the scientific realm but rather came to the light of day as an “economic” edge.
Dr. Krummel: I would not underestimate the talent and creativity of those that we train who go out into private practice. Much innovation has come from very active practitioners.
Dr. Clayman: Right, but they do not have the infrastructure that we are blessed with at universities both to create and to validate.
Dr. Schauer: I agree that academia does not have a monopoly on creative ideas. But perhaps academia should play a major role in defining validation-type studies. That is one area where we may be especially well suited to meet an important need.
THE INNOVATION-TRAINING INTERFACE
Question from audience: I have a dilemma as a residency program director. Our residents want to learn the new technology—laparoscopic surgery, robotic surgery, etc—but we have them in our program for such a limited time. How do you justify teaching them new technology and at the same time still teach them basic, traditional surgical procedures, especially with the reduction in residents’ hours? It is fine to be able to do a nephrectomy laparoscopically, but if you get in trouble, you still have to know how to do a good open nephrectomy. How do you address this?
Dr. Schauer: I think the answer largely is fellowship training. Emerging procedures probably should be introduced in fellowship programs until they reach the point where they are so standardized that they become a major part of practice. For example, cholecystectomy quickly became part of general surgery practice, but laparoscopic colectomy took several years to evolve and was taught primarily in fellowship or advanced training, after which it gradually filtered down to residency programs.
Dr. Krummel: All of us who are responsible for training are wrestling with this problem. Residents are expected to learn more yet do so in less time. One approach would be early specialization, so that instead of 5 years of general surgery, you would have 3 years of general surgery and then 3 years of, for instance, thoracic surgery. Also, Ralph mentioned earlier the advantage of skills labs. We increasingly see that type of approach as a backbone for providing broad training without putting patients at harm.
As for teaching the use of new technology, first you have to teach the existing base of practicing surgeons. Here again there is much to be said for skills labs, and I give credit to the American College of Surgeons for its drive to establish and accredit centers around the country as a way to teach this base of surgeons.
Dr. Schauer: If I may expand this question beyond residency and fellowship training, how do we balance the desire to share new innovations with our colleagues against the need to temper their desire to prematurely jump into an area where they do not yet really belong? Chris, I know this applies to your challenges in disseminating knowledge about NOTES.
Dr. Christopher Thompson: Yes, courses on NOTES are also being held in conjunction with all the major society meetings, and we are seeing many enthusiastic trainees at these hands-on courses. The original intent was to give attendees instruction on setting up their own animal labs, yet some trainees took it beyond this limited purpose. As a result, some in our field believe that we should not allow foreign physicians to come here to be trained in NOTES, for fear they will go back to their home countries and use it on humans. I am not certain that that approach is the best way to go, but there has been much discussion about how to handle this. It is a real conundrum. Certainly there are a number of surgical residents and gastroenterology fellows who are clamoring to get into the lab right now and learn these techniques.
Dr. Clayman: This goes back to our earlier discussion about from where new technologies should emerge. What frightens me are the consequences of creative activity occurring outside the university, where there are no laboratories or animal or cadaver models for refining or testing a technique. To me, it was frightening to see laparoscopic cholecystectomy suddenly emerge as a craze without the proper animal and clinical studies having been done. That is not the way I believe clinical research should go forward. I once heard a prominent urologic surgeon say at a major surgical meeting, after a presentation on the impact of percutaneous stone surgery on the canine kidney, “Now that I’ve done a thousand of these in humans, it’s reassuring to know that it’s safe to do in dogs.” That is not the way it should happen, and every time it does happen that way, we pay a large price, some of us as individuals and all of us as a society.
Dr. Cooper: The answer therefore is to use our academic facilities to facilitate the training of those in community practice. We should continue to offer training because we have the resources to make it available.
Dr. Clayman: Yes, and this is why I emphasized earlier that support for surgical training centers is so essential. I see all the dollars spent on health care advertising and wonder why these dollars are not instead poured into surgical training, or research facilities, or training simulators.
The way we should train surgeons in new technologies is to train them on simulators equipped with a properly vetted curriculum. This is the future for training, because once you put instruments through small ports, everything becomes measurable—economy of motion, past pointing, and efficiency; simulators with a curriculum will also be able to assess the trainee’s cognitive abilities. When an individual performs well on the simulator, he or she can then come into the operating room and work with surgeons experienced in the procedure. The use of simulators in this manner should ultimately improve the overall quality and safety of each surgical specialty.
RISE OF THE ROBOTS
Question from audience: I am curious how the panel members interpret early randomized trial data showing an increased cost without an improvement in care with the use of surgical robots in certain procedures. Should we persist or consider an investment in the future as robotic technology improves and surgeons further adapt to it?
Dr. Cooper: I think the robot should be used only for those procedures for which it has unique capability and can perform a task better than we can. It appears that the robot performs better than the ear, nose, and throat surgeon for operations on the base of the tongue. The same may be true of prostate surgery, but I am not certain. But to do a laparoscopic Nissen repair with a robot…as Dr. Nat Soper of Northwestern University has said, “If I needed a robot, I shouldn’t be doing laparoscopic Nissens.”
The robot provides light, it gives you magnification, and it reduces tremor. We should concentrate on its use for operations where these attributes are particularly valuable. But we should be wary of its use as an expensive marketing tool.
Dr. Clayman: The robot provides you with superhuman capabilities: 10 to 30X magnification, no tremor, a 540-degree wrist, instrumentation with 6 degrees of freedom, and motion scaling. It allows you to be a better surgeon than you are without it. I agree that it is expensive. It is woefully overpriced at this point, but I believe the expense will come down with time. It is no different than the first computers, which were terribly expensive. The robot enables surgeons to do a better job than they would without it if we are talking about reconstructive-type surgery.
Ergonomically, the robot is very positive for the surgeon. For the first time, the surgeon is actually allowed to sit down in a comfortable environment and can work for 4 hours straight, get up at the end of the surgery, and feel fine. If you are older than 50 and you operate standing at the table staring at a television screen on the other side for 4 to 6 hours, you are going to ache afterwards. I believe surgeons work better if they are comfortable.
Dr. Schauer: At least within my field of general surgery, there has been no evidence that this superhuman ability has translated into superhuman results, in terms of reduced operating time, fewer complications, or better efficacy. We should probably develop the metrics to measure progress. How do the theoretical benefits translate into clinical benefit?
Dr. Clayman: It is not theoretical in radical prostatectomy if you look at the data. The potency rates for patients who undergo robotic surgery for these procedures are now almost 90%, which is something that no surgeon performing open prostatectomy has ever achieved. Fortunately, the continence mechanism is so strong in most adults that it does not matter whether prostatectomy is done with a robot or open surgery—patients are probably going to be all right. But the bottom line is that robotic surgery is a bit better. Most surgeons would use it if it were free. The problem is that it is so expensive right now and it is breaking the backs of many hospitals.
Dr. Schauer: You make a good point. Demonstrating metrics is important, and prostatectomy is a good example. But I am not aware of any other procedures for which benefit from robotic surgery has been documented.
Dr. Krummel: The history of robotic surgery is so interesting because the killer application was supposed to be coronary work—percutaneous bypass surgery. But then the heart port went to pot and patients with anterior wall lesions ended up not being a big enough group. It turns out that it is still difficult to do and there is not a lot of room. So prostatectomy has ended up as the initial killer application.
Keep in mind that the current robot is not an end device. We will see more. There are now robotic steerable catheters that I think will be adopted into NOTES procedures. This theme of immediate benefit versus follow-on iterations is the story of device development in this country.
How should we introduce and evaluate new procedures?
By Joel D. Cooper, MD
Time magazine published an article in 1995 titled “Are Surgeons Too Creative?” that examined the question of whether operations should be regulated the way that medications are.1 The piece featured two patients. One, a patient with emphysema who underwent lung volume reduction surgery at our institution during the early days of this procedure, had a good outcome. The other was a neurosurgical patient who had a bad outcome.
The public is somewhat sympathetic to this article’s premise, which can be viewed as a call to require a similar level of evidence for surgical procedures as for new drugs. This sympathy arises from the expense of new technologies, pressure from payors to control costs and increase profits, hospital budget restraints, and the reality of increasingly well-informed patients.
Yet there are distinct differences between drugs and surgery. A new drug does not change over time. A new drug is associated with a variable biologic response whose assessment often requires large numbers of patients and considerable follow-up. And a new drug may manifest unforeseen late side effects and toxicities far removed from the time of initial use. In contrast, none of these characteristics applies to surgical procedures. A surgical intervention changes over time as the technique and experience evolve and as refinements are made in patient selection and in pre- and postoperative management. With this evolution comes a change in risk over time. Patient selection for surgery is as much an art as a science; each patient requires assessment of both the potential benefits and risks of the procedure, which argues against offering an operation by prescription. Moreover, with surgery, the facilities and the operator’s skill and experience levels vary from one center to another.
INTRODUCTION OF NEW PROCEDURES: COVERAGE VS VALIDATION
Introduction of a new surgical procedure depends on the nature of the procedure and the other interventions that may be available for the condition. In assessing how new procedures should be introduced, I believe we need to distinguish between coverage and validation. Coverage—ie, payment for the procedure—is an economic issue, whereas validation involves an ethical and scientific evaluation of the role of the procedure.
Coverage by an insurer should have at least theoretical justification and presumption of benefit. For instance, the rationale behind a heart transplant for a patient with a failing heart is obvious. Coverage generally requires preliminary evidence of efficacy, possibly in an animal model, although no animal models may exist for some conditions. Most important, a different standard for providing initial coverage should be applied if no alternative therapy exists for a condition that is severe, debilitating, and potentially life-threatening; if a new procedure treats a condition for which a standard therapy already exists, the standard for coverage must be higher. Finally, coverage in all cases should require ongoing reassessment of the procedure.
In contrast, validation is a scientific analysis of results over time, including long-term results, and can be accomplished by well-controlled case series, particularly if the magnitude of the benefit is both frequent and significant and especially if no alternative therapy exists. Randomized clinical trials are the gold standard for appropriate interventions but are not always applicable.
A 1996 study by Majeed et al2 provides a good example of validation-oriented surgical research. In this blinded trial, 200 patients scheduled for cholecystectomy were randomized to either laparoscopic or open (small-incision) procedures. The study found no differences between the groups in terms of hospital stay or postprocedure pain or recovery. In an accompanying commentary,3Lancet editor Richard Horton praised the design and conduct of the study, noting that it was very much the exception in surgical research, which he argued was preoccupied with case series. Horton offered the following speculation about this preoccupation:
Perhaps many surgeons do not see randomised trials as feasible strategy to resolve questions about surgical management. Cynics might even claim that the personal attributes that go to make a successful surgeon differ from those needed for collaborative multicentre research.3
IS THE ‘SURGICAL SCIENTIST’ AN OXYMORON?
Barnaby Reeves, writing in The Lancet 3 years later, offered a more diplomatic take on the difficulty of evaluating surgical procedures:
What makes a surgical technique new is not always easy to define because surgical procedures generally evolve in small steps, which makes it difficult to decide when a procedure has changed sufficiently to justify formal evaluation.4
Reeves went on to argue that doing an evaluation too early may preclude acceptance, since the technique may not have evolved sufficiently and surgeons may not have mastered it; conversely, doing an evaluation too late may make the evaluation moot, since the technique may have already become established and withholding it may be deemed unethical. Additionally, he noted that the quality of surgical evaluation is complicated by the possibility that some surgeons have better mastery of—and therefore better outcomes with—one procedure while other surgeons have better mastery and outcomes with an alternative procedure.4
These concerns were well captured by the late Dr. Judah Folkman, whom I once heard say, “When a basic scientist is informed that another investigator cannot reproduce his work, it has a chilling effect; for the surgeon, however, it is a source of pride.”
RANDOMIZED TRIALS VS CASE SERIES: A TIME AND PLACE FOR EACH
Even as we recognize these challenges specific to surgical evaluation, we are still left with the task of determining when a randomized controlled trial is appropriate and when a case-control series may suffice.
There are three broad sets of circumstances in which a randomized trial is essential:
- For preventive procedures, ie, when the operation is done to reduce the potential for a future adverse event. An example would be evaluating carotid endarterectomy to reduce the potential for stroke in asymptomatic patients with 60% or greater stenosis. Only a randomized trial could have shown a difference in favor of endarterectomy over aspirin plus best medical therapy.
- To compare a procedure with alternative medical or surgical interventions. I would argue that laparoscopic surgery should have been introduced with randomized trials, as it begs one to suspend judgment and accept that small incisions are invariably and de facto better than a large incision.
- For trials in oncology, where the outcome depends on long-term results, such as survival or time to recurrence. Examples would include comparisons of surgery alone versus surgery plus chemotherapy for prevention of cancer recurrence.
Similarly, there are several scenarios in which a case-control series is appropriate and adequate:
- When no alternative therapy exists. Falling into this category, in my view, are lung transplant, which we introduced successfully at the University of Toronto in 1983, and lung volume reduction surgery, which we introduced in 1993.
- When the natural history of the condition is well documented and the impact of the intervention is obvious.
- When the magnitude of the procedure’s effect is measurable, significant, and expected.
RANDOMIZED TRIALS IN SURGERY
Advantages of randomized trials
Randomized clinical trials confer a number of advantages. They eliminate bias. They ensure a balance between treatment groups in terms of known or unknown prognostic factors. And, importantly, they have a major impact on payors.
A tale of two Medicare payment decisions
The impact of clinical trials on payors is exemplified by the contrasting stories of two procedures: transmyocardial laser revascularization and lung volume reduction surgery.
Transmyocardial laser revascularization (TMR) involves the creation of channels in the myocardium with a laser to relieve angina. Although TMR is a dubious intervention with no physiologic rationale (similar to internal mammary artery ligation for angina5) and no proven improvement in life expectancy (only a reduction in pain), it was approved for reimbursement by Medicare because it was investigated in a randomized trial.6 However, the “randomized trial” was not truly a randomized investigation because the control patients received only medical therapy and did not go to the operating room to receive a sham operation.6 Despite this flaw, the perceived authority of the trial was sufficient to influence Medicare.
In contrast, Medicare refused to pay for lung volume reduction surgery until it was subjected to a randomized trial, despite the fact that the procedure had produced tremendous benefit in hundreds of patients at multiple centers who otherwise could not have achieved such benefit. Only after $50 million was spent on a randomized controlled trial, the National Emphysema Treatment Trial (NETT),7–9 did Medicare agree to pay for lung volume reduction surgery. The trial showed that over 5 years, the procedure was associated with significant improvements in life expectancy, exercise tolerance, and quality of life, but the study took 8 years to conduct and by then it was a bit too late, as detailed in the following section.
NETT: A case study in how a trial can be counterproductive
Lung volume reduction surgery is an operation based on the recognition that the crippling effects of emphysema are hyperinflation of the chest, flattening of the diaphragm, and inability to move air in and out of the chest. The notion that the chest can be reconfigured in the patient with emphysema by removing the distending overinflated emphysema led us to develop the volume reduction operation.
The NETT was initiated by Medicare, and the protocol denied compassionate crossover of patients.7 In an attempt to establish clinical equipoise, surgeons who participated were not allowed to perform any volume reduction operations on non-Medicare patients or on Medicare patients not enrolled in the trial. After 2 years of slow patient enrollment, the clinical trial committee, in an effort to increase enrollment, eliminated the original entrance criteria specifying certain degrees of hyperinflation and diffusing capacity. An excess of mortality was discovered 2 years later in a subgroup randomized to volume reduction surgery;8 not surprisingly, further analysis showed that the excess mortality was largely confined to patients who would have been excluded based on the original entrance criteria. This is a matter of public record but was never acknowledged in published reports of the trial. Final 5-year NETT results showed that in patients with upper lobe emphysema, lung volume reduction surgery improved survival, increased exercise capacity, and improved quality of life.9 By the trial’s completion, however, the procedure’s reputation had been tarnished irreparably by bad publicity from the deaths attributable to the misguided changes to the original eligibility criteria.
Disadvantages of randomized trials
The NETT exemplifies many of the drawbacks of randomized trials in surgery, particularly the need to wait long periods while they are being conducted. During the 8 years in which the NETT was ongoing, the number of lung volume reduction operations declined, with the typical center performing fewer than 6 cases per year, on average. That limitation is certainly not conducive to the development of a new procedure for a disabling condition in patients with no ready alternative.
Other disadvantages of randomized trials in surgery are their considerable expense and the fact that they often are not generalizable and often are not appropriate. Moreover, when they are flawed, randomized trials propagate, sometimes for decades, misleading information that is nonetheless considered “authoritative.” For instance, lung cancer kills more men and women in the United States than the next three cancers combined, yet, on the basis of a flawed randomized trial,10 the American Cancer Society advises smokers to wait for symptoms before undergoing chest radiography, instead of recommending annual screening chest radiography. This is a major reason why two-thirds of lung cancer cases are discovered too late to save the patient.
‘Better to know nothing than to know what ain’t so’
Indeed, this potential for randomized clinical trials, when flawed, to propagate misleading information makes the perceived authoritativeness of randomized trials both an advantage and a disadvantage. As Berger and colleagues noted a few years ago, overuse of randomized trials for evaluating emerging operations could have led to the demise of heart transplantation, mechanical circulatory assist devices, cardiac valve procedures, coronary bypass grafting, and repair of congenital lesions.11
For this reason, one of our responsibilities when reading the literature and conducting studies is not just to answer unanswered questions but to question unquestioned answers. As 19th-century humorist Josh Billings put it, “It’s better to know nothing than to know what ain’t so.”
A PERSONAL PERSPECTIVE
In my view, health care providers should restrict the application of new procedures to a limited number of centers of excellence that have appropriate resources and experience. Those centers should be required to document and report specified information regarding morbidity, mortality, and objective measures of outcome; if they do not comply, they should lose the privilege of doing such research. The data should be reviewed by an independent, nongovernmental scientific panel. In this way, the procedure can be offered to appropriate patients, insurers and patients can be protected against abuse, and the necessary data can be collected for objective analysis.
Idea to implementation: A personal perspective on the development of laparoscopic nephrectomy
By Ralph V. Clayman, MD
Change in the surgical world involves three aspects, which I refer to as the three Ds: discovery, development, and dissemination. Change requires proof that the new method is superior to the old. When we, as innovators, develop something “new,” I believe that our immediate subsequent task is to do everything we can to prove that this “new” finding is of no value whatsoever before we determine that it is worth advancing.
Getting to Malcolm Gladwell’s tipping point—the act or event after which nothing is ever the same— requires a team of people, usually from different disciplines, coming together to concentrate on a problem, or an individual whose experiences in different fields provides the ability to “see” the next level. In my opinion, one person working in one discipline rarely leads to breakthrough progress in medicine.
These observations about surgical innovation stem largely from my experience in the development of laparoscopic nephrectomy, in which I was privileged to play a role while at Washington University in St. Louis, which I will outline here.
THE HISTORY BEHIND LAPAROSCOPIC NEPHRECTOMY
After doing preliminary work in dogs, the German surgeon Gustav Simon performed the first human nephrectomy in 1869, in a woman with a ureteral vaginal fistula. The operation was a success: it took him 50 minutes to complete the procedure, and 6 months later the patient went home.
From that point in 1869 until 1990, progress in nephrectomy was minimal, with open surgery remaining the gold standard. While the surgeon’s tools remained largely unchanged, the advances that did occur were in anesthesia, analgesia, and antibiotics, which allowed patients to better survive the onslaught of the operation.
In an unrelated arena, laparoscopy was developed in 1901 by another German surgeon, Georg Kelling, who pumped air into the peritoneal cavity of a dog in a successful effort to stop bleeding from the stomach. Within the pneumoperitoneum, Kelling was able to examine the canine organs with a cystoscope at pressures as high as 140 mm Hg. This discovery was not applied clinically, however, until 9 years later when the Swedish gastroenterologist H.C. Jacobeus used Kelling’s pneumoperitoneum concept and a cystoscope to visualize the peritoneal cavity to search for cancer. The technique advanced little in the subsequent decades —apart from Semm’s seminal laparoscopic removal of the appendix in the 1960s—until 1985, when the first laparoscopic gallbladder removal ignited the era of laparoscopic cholecystectomy.
Three technological developments spurred this recent surge in laparoscopy: (1) the ability to affix a camera to the endoscope, (2) the ability to display the camera’s images on a video screen, and (3) the development of self-feeding clip appliers to allow occlusion of vascular or ductal structures.
THE EVOLUTION OF LAPAROSCOPIC NEPHRECTOMY
Discovery
I became interested in the possibility of laparoscopic nephrectomy during the laparoscopic cholecystectomy craze in the late 1980s. At that time, I was working with Dr. Nat Soper, performing laparoscopic cholecystectomies in pigs to show that the procedure could be done safely with electrocautery rather than a laser. As it turns out, the anatomy of the porcine kidney is such that the colonic reflection lies medial rather than lateral to the organ. As such, the kidney is quite visible as soon as one enters the abdomen. Indeed, the kidney seemed to be saying to us, “Hey, what about me? I could come out through that hole too.” That is basically how the idea arose.
So, along with Dr. Lou Kavoussi and many others in our research team, we attempted laparoscopic nephrectomy in the pig and succeeded: the kidney could be removed through a small hole by entrapping it in a sack, breaking it up in the sack, and pulling it out.12 The team involved in this discovery were specialists in urology and general surgery as well as biomedical engineers from industry, specifically a team from Cook Urological led by Mr. Fred Roemer.
After performing this technique numerous times in the laboratory, we reduced the operation’s duration to 90 minutes, at which point we believed the procedure had advanced sufficiently to be considered for clinical use.
Development
The patient we selected for the initial clinical case was an 85-year-old woman with a 3-cm mass in her kidney. She was deemed to be “too sick to operate on,” so she was presented to me as a candidate for the new laparoscopic procedure.
Amazing as it may seem in our current medical climate, at that time (1990) we were faced with the question of whether or not to seek institutional review board (IRB) approval. The argument could be made that since radical nephrectomy had been practiced for 120 years and laparoscopy had been around for nearly 100 years, the combination of these two well-accepted procedures might require nothing more than physician-patient informed consent. However, the concept of “informed consent” in this context was problematic: what could we tell the patient about a procedure that had never been done before except that if it was not working out we would convert to the standard open procedure?
A senior colleague—actually my boss at that time, Dr. Bill Catalona—sagely advised me to get IRB approval, noting, “If the operation works out well, you’ll be fine, but if it doesn’t work out well, they’ll kill you if you don’t have approval.” So we fortunately ended up seeking (and receiving) IRB approval, as well as providing, as best we could, informed consent to the patient and her best friend.
Our next consideration was designating a team member to determine if and when conversion to open surgery would be necessary. We needed a “referee” to aid in objectively determining a point at which we should convert. For our team, that person was Dr. Teri Monk, our anesthesiologist, who had no previous experience with our laboratory work but understood what we were attempting.
So we proceeded with the first clinical laparoscopic nephrectomy on June 25, 1990. The kidney was embolized the morning of the procedure. Five laparoscopic ports were placed. The clip appliers proved too small for renal vein occlusion, so the main renal vein had to be traced to its branches; in total we clipped five separate sets of renal vascular branches. The kidney was ensnared and morcellated, which took 7 minutes. Total operative time was 6.8 hours. The complications that arose were not anticipated:
- Intraoperative oliguria due to the prolonged pneumoperitoneum
- Fluid overload (postoperative congestive heart failure) due to providing fluids to the patient as though this were an open procedure
- Dilutional anemia, again due to providing excessive fluids for a closed procedure.
Postoperative pain medications consisted of one dose of morphine sulfate. The patient was discharged on postoperative day 6 and resumed normal activities by postoperative day 10.13
Dissemination
Before a new procedure is disseminated, evidence of the four Es—efficiency, effectiveness, equanimity, and economy—must be obtained. In retrospective reviews, laparoscopic removal was associated with a slightly longer operating time but much less blood loss, a shorter hospital stay, and fewer complications. The immediate cancer cure rate was the same for open and laparoscopic nephrectomy, and over time the laparoscopic procedure has been shown to be just as good as open surgery at 5 and now 10 years. Also, with time, laparoscopic nephrectomy was shown to reduce institutional costs.
The next question was the proper way to disseminate this knowledge. At Washington University we took the traditional route of providing courses, offering 17 courses on laparoscopic surgery to nearly 1,000 urologists from 1985 to 2002. But as Winfield and associates later showed, only 54% of urologists who completed a 2.5-day hands-on, laboratory-based laparoscopic course actually ended up introducing laparoscopy into their practice.14
The challenge of dissemination is still with us, and we need to find better methods of transferring new skills to our surgical colleagues. In this regard, longer experiences, such as weeklong mini-fellowships and the development of procedure-specific surgical simulators, hold great promise.
UNANSWERED CHALLENGES, UNMET NEEDS
With the advent of any technology comes a cornucopia of unanswered questions and challenges. In the areas of discovery and development, a key question is whether every procedure performed using a new Food and Drug Administration (FDA)-approved technology requires a separate approval by the IRB and ethics committee. For instance, if robotic prostatectomy is approved and performed, are separate approvals needed for robotic nephrectomy, robotic pyeloplasty, and robotic vasectomy? Where would or should the approvals end?
With respect to dissemination, many questions remain: How is a new technology taught effectively? How is surgical competency tested? How is clinical performance or proficiency evaluated?
One problem specific to dissemination is a lack of funding. While ample funding is available for discovery and development, as they bring prestige and profit, dollars are scarce for dissemination, or the teaching and testing of competency and proficiency with new procedures.
Evidence of our failure to educate the postgraduate surgeon abounds in terms of poor outcomes and malpractice suits. The response of government all too often is the knee-jerk reaction to protect (ie, regulate), not educate. To be sure, we can do better, but only if our society commits to the process—not with words, but with funded educational action.
With regard to the last, I believe there is an unmet need for the development of accurate, validated surgical simulators. As a society, we need to find a way to fund the development of simulators for each surgical subspecialty and then use these devices to objectively test an individual surgeon’s manipulative skill as well as cognitive ability when he or she seeks certification or recertification—and perhaps, albeit in an abbreviated 5-minute format, before beginning each operative day. We owe this to ourselves, but most of all to our patients, who in all confidence place their lives in our hands.
Special perspectives in infants and children
By Thomas M. Krummel, MD
If we surgeons take a step back and consider for a moment what has changed in the operating room (OR) in the past 50 to 60 years, the clear answer is, “Just about everything.” The monitors, pumps, transport devices, and OR tables and lights have all changed dramatically, as have the tools, catheters, sutures, energy sources, scopes, staplers, ports, valves, and joints. If we consider technologies outside the OR that guide what we do inside the OR, the changes are just as striking. Circulatory assist devices for the failing heart and widespread use of dialysis for the failing kidney postdate 1950, as does all of our modern imaging capability—ultrasonography, computed tomography, magnetic resonance imaging, positron emission tomography, functional imaging. As for pharmacotherapy in 1950, there were three antibiotics, no antivirals, one antifungal, and three chemotherapeutic agents. Open drop ether was the anesthetic of choice. Not only have the tools and technologies changed, but virtually every procedure has been changed. Both our profession and the industry that has developed these devices and tools can be rightfully proud.
It is likewise necessary to recognize that our patients have been partners in this innovation. Many of them have given informed consent to participate in research and experimental procedures with the expectation that the benefits might accrue only to future patients and not to themselves. That is a hell of a contribution, and we can be proud of our patients’ partnership.
THE GOOD, THE BAD, AND THE UGLY OF INNOVATION
The history of progress in surgical care is always about innovation, and such progress almost always begins with an unsolved patient problem, regardless of the solution that is developed, be it a tool, a device, a technology, or a surgical procedure. At the same time, any discussion of the ethics of surgical innovation should recognize that while efforts to solve patient problems over the years have had many good results, they have also had some bad results and even the occasional ugly result.
This conference has already focused on much of the good that has come from surgical innovation, including transplantation, remarkable advances in cardiac and gastrointestinal surgery, a host of devices, and too many other benefits to list. Yet missteps have been made along the way, such as bloodletting, gastric freezing as a therapy for ulcer, and carotid denervation for treatment of asthma in children.
Then there are the ugly incidents, and these notably include a number of cases involving children, an issue of special interest to me as a pediatric surgeon. Consider the following examples:
- Edward Jenner’s notorious cowpox experiment in the late 18th century was conducted in an 8-year-old boy.
- A well-documented literature shows that orphans were used as subjects for tuberculosis and syphilis inoculations.
- The more recent case of Jesse Gelsinger involved a teenager with a nonlethal condition who died in a clinical trial of gene therapy, after which an undisclosed financial interest on the part of one of the treating physicians was revealed.
It should give us pause to note that many of these practices that look foolish in hindsight probably seemed more rational at the time they were undertaken.
CHILDREN: THE ORPHANS OF INNOVATION
Children have been the orphans of innovation, as technology development specifically for children has traditionally been a low priority. There are several reasons for this:
- FDA standards for approving therapies in children are high. For instance, the vast majority of chemotherapeutic drugs are not approved for use in children because conducting a trial specifically in children is deemed too expensive.
- Pediatric markets for therapies are small.
- The payor mix is poor.
The benefts of duality
Nevertheless, children have benefited enormously from the duality of technology development, in which a technology developed for one population—either adult or pediatric—ends up benefiting both populations. For instance, no one would have invented the pulse oximeter to care for a child, yet now it is the only device with which infants and children are monitored in the operating room and during transport.
Likewise, in some cases the solutions to pediatric problems have had reciprocal benefits in adults. Ligation of the patent ductus arteriosus and the Blalock-Taussig shunt for tetralogy of Fallot opened the door to our understanding of surgery on the great vessels and ultimately enabled the development of cardiac surgery. Similarly, the early impetus for Thomas Starzl’s groundbreaking work in transplantation was focused on children with biliary atresia even though this work is now much more widely applied in adults.
ETHICAL PRINCIPLES APPLY EQUALLY TO ADULTS AND CHILDREN
The principles of medical ethics that began with Hammurabi in 1750 BC and progressed through Hippocrates’ work circa 400 BC, the 1946 Nuremberg medical trial, the 1964 Declaration of Helsinki,15 Henry Beecher’s classic exposé in 1966,16 and the 1979 Belmont Report17 are just as valid for children as they are for adults.
Francis Moore, the great surgeon who created the environment and the team at Brigham and Women’s Hospital that facilitated the first twin-twin transplant, identified six important components of ethical surgical innovation:18,19
- A solid scientific background (basic laboratory research)
- A skilled and experienced team (“field strength,” as Moore called it)
- An ethical climate within the institution
- An open display for ongoing discussion
- Public evaluation
- Public and professional discussion.
The principles behind these components remain as true today as they were 20 years ago when Moore outlined them.
SPECIAL CONSIDERATIONS IN PEDIATRIC SURGERY: A CASE STUDY IN MATERNAL-FETAL MEDICINE
The Belmont Report, mentioned above, was developed by the US government in 1979 to form the basis of regulations for federally funded research involving human subjects.17 The report identified three basic principles that must underlie such research:
- Respect for persons—protecting the autonomy of all subjects, treating them with courtesy, and allowing for informed consent
- Beneficence—maximizing benefits from the research initiative while minimizing risks to the subjects
- Justice—ensuring reasonable, nonexploitative, and well-considered procedures that are administered fairly.
In pediatric surgery, everyone agrees that the “best interests of the child” must be protected, but the issue of autonomy (a key element of the first Belmont principle) is more difficult to define, of course, when the patient is a child rather than an adult. The question of autonomy is especially tricky in the evolving field of maternal-fetal medicine: what if the patient is a fetus and the mother is an innocent bystander?
Over the past 20 years, tremendous progress has been made in our understanding of diseases of the fetus, particularly diseases that limit fetal viability and diseases that cause serious organ damage but which may be more responsive to postnatal therapy if they are treated prenatally. Michael Harrison, N. Scott Adzick, and a few of their disciples have laid the ethical groundwork for consideration of the fetus as a patient.
Considerations in maternal-fetal medicine
I will conclude with a case in maternal-fetal medicine for us to consider and perhaps debate in the panel discussion at the end of this session. As you consider this case, keep in mind several important observations relating to maternal-fetal medicine:
- The mother’s health interests cannot be underestimated.
- Most “fixable” fetal lesions (ie, those that interfere with development and cannot be fixed postnatally, but for which intervention in utero may result in normal development) are very rare. They include obstructive uropathy, lung lesions causing hydrops, congenital diaphragmatic hernia, sacrococcygeal teratoma, hydrocephalus, twin-twin transfusion syndrome, congenital high airway obstruction, hydrothorax, myelomeningocele, and congenital heart disease.
- The field is evolving, and the efficacy of therapy is supported by variable level I, II, and III evidence.
- The law has not kept (and perhaps cannot keep) pace with developments in this field.
Case study
A 24-year-old healthy woman has a fetus of 28 weeks’ gestational age with progressive lower urinary tract obstruction with megacystis, bilateral hydronephrosis, and oligohydramnios. In other words, there is diminished volume in the uterine cavity that causes compression of the fetal chest and subsequent respiratory compromise that will be fatal if not addressed. The karyotype is a normal 46,XY male. Serial urine sampling reveals electrolyte and protein profiles with a good prognosis.
Prenatal counseling with fetal therapy specialists suggests that this is the “perfect case” for a vesicoamniotic shunt. This is the least invasive, most successful fetal surgical intervention. It is done under local anesthesia and involves transabdominal transuterine percutaneous placement of a double-lumen pigtail catheter in the fetal bladder. There has never been a reported maternal death, and morbidities have been minimal. Renal and pulmonary function both are improved by approximately 80% in fetuses treated with this intervention, and survival is improved.
The father is eager to proceed. The mother is ambivalent. Should the mother be pressured to proceed, for the good of the child?
Questions to ponder
The following questions are intended to be provocative, with no clear-cut answers:
- Should (or does) the fetus have independent moral status? Is it full, graded, or none? Does it matter?
- What are the beneficence-based obligations to the fetus? At 28 weeks’ gestation, the fetus is viable outside the uterus. The fetus is otherwise well, without a lethal karyotype, and has currently good renal function.
- What are the beneficence-based and autonomy-based obligations to the mother? What are the mother’s obligations to the fetus?
- What if the mother ultimately decides to proceed and the insurance company denies coverage? What are the social responsibilities to care, cost, and research?
These questions lend themselves to discussion. As much as we surgeons like to be certain about what we do, we would do well to heed the quote from Voltaire that the great surgeon Norman Shumway hung on his office door: “Doubt is not a very agreeable state, but certainty is a ridiculous one.”
Bariatric surgery: What role for ethics as established procedures approach new frontiers?
By Philip R. Schauer, MD
Obesity is a staggering problem: 100 million Americans are overweight, 85 million more are obese, and another 15 million are morbidly obese (ie, ≥ 100 lbs above ideal body weight). The incidence of obesity is rising rapidly and threatens to shorten the life spans of today’s young generations relative to their parents. Unlike other conditions, such as cardiovascular disease and cancer, obesity has seen no widespread progress in management in recent years.
Recognition of obesity as a medical problem is a challenge in itself. Many people consider obesity to be a character flaw or a behavioral issue and fail to recognize it as a disease entity. Yet obesity is the root cause of many metabolic conditions and diseases with metabolic components, including type 2 diabetes, heart disease, blood pressure, metabolic syndrome, acid reflux, gout, arthritis, and sleep apnea.
The approach to obesity treatment can be conceptualized as a pyramid, with the aggressiveness of the intervention based on the patient’s body mass index (BMI). At the base of the pyramid, for patients with lower BMIs, are minimally invasive (and minimally effective) interventions involving changes in diet, physical activity, and other lifestyle factors. As BMI increases, so does the intensity of treatment, to include pharmacotherapy and eventually bariatric surgery. Traditionally, surgery has been considered only at the very top of the pyramid, for morbidly obese patients, and is usually not offered as an option for the vast majority of people with this condition.
The sad reality is that the various combinations of these therapies are effective in fewer than 1% of the approximately 100 million Americans who are obese. Because surgery has been shown to be the most effective therapy for obesity, the remainder of my discussion will focus on surgery, with an eye toward potential new indications for bariatric procedures and the questions they raise.
SURGICAL APPROACHES TO OBESITY
Bariatric surgery has evolved over the past 50 years. Although there are about a dozen different permutations of bariatric procedures performed in the United States today, they fall into one of three major types of operations, as outlined below:
Gastric banding reduces appetite and satiety by adjusting and tightening the gastric band. This procedure has been in existence for 10 to 15 years and represents about 25% of operations for obesity in the United States.
The biliopancreatic diversion procedure diverts most of the small bowel and radically reduces absorption of calories. Patients undergoing this procedure lose weight because few calories are absorbed into the body. This approach, while quite effective, is somewhat radical and represents only about 2% of the operations for obesity in the United States.
The Roux-en-Y gastric bypass procedure has been the dominant procedure over the past 15 to 20 years. A combination of the above two procedures, it involves reducing the gastric reservoir and bypassing the stomach and upper intestine. The reduction in gastric volume reduces calorie intake by enhancing satiety, and the limited foregut bypass moderately reduces absorption.
No randomized trials, but much support from observational studies
Virtually none of these procedures evolved with randomized controlled trals. Instead, they evolved incrementally, primarily on the basis of knowledge gained from case procedures. Despite the lack of randomized trials, these operations have been shown to be effective, particularly in patients with multiple metabolic abnormalities associated with severe obesity. A large body of data from case-control and cohort studies demonstrates not only dramatic improvement in metabolic abnormalities with the use of various bariatric procedures, but also improvements in quality of life and survival.20–26 The two most recent of these studies, published in 2007, found reductions in mortality of 29% (adjusted) and 40% among surgical patients compared with well-matched obese controls during mean follow-up of more than 7 years.25,26 Reductions in the incidence of cardiovascular mortality and, secondly, cancer-related mortality were the two major contributors to the overall mortality reduction in these two studies. Consistent with this latter finding, obesity is starting to be thought of as a disease that may lead to cancer.
NEW FRONTIERS FOR BARIATRIC PROCEDURES
The current indications for bariatric surgery have existed intact for about 25 years, and were based on limited evidence available at the time. They are basically as follows, assuming acceptable operative risk and appropriate patient expectations:
- BMI greater than 40 kg/m2
- BMI greater than 35 kg/m2 with significant obesity-related comorbidities.
Payors adhere strictly to these indications, such that they will not pay for bariatric surgery in a patient with a BMI less than 35 kg/m2. This raises questions about the appropriateness of such a firm threshold and whether expansion of these strict indications may be reasonable.
Even without broadened indications, the volume of bariatric procedures in the United States has grown dramatically in recent years. Whereas only 10,000 to 20,000 of these operations were performed annually in the 1990s, approximately 200,000 such procedures were performed in 2007, and this number is expected to double over the next 5 years or so.
This growth in volume has been paralleled by burgeoning media interest in bariatric procedures, particularly in the last few years. More attention can be expected as we increasingly recognize the potential of bariatric procedures for indications beyond strictly the treatment of morbid obesity. At least two new frontiers loom: metabolic surgery and endoscopic surgery.
Metabolic surgery
Procedures that incorporate a bypass—the Roux-en-Y gastric bypass and the biliopancreatic diversion —have been associated with a reversal of metabolic diseases such as type 2 diabetes.27–32 Many patients with type 2 diabetes who have undergone these procedures have been able to be weaned off insulin and insulin-sensitizing medications while maintaining normal blood glucose levels. The effect has been profound and immediate, occurring even before the patient loses weight. In one series of patients with type 2 diabetes who had undergone a bypass operation, 30% left the hospital in a euglycemic state.29
These observations have been made primarily in the morbidly obese population, who are the primary candidates for bariatric bypass procedures. However, because of the rapid improvement in metabolic abnormalities that has been observed, interest has arisen in applying these procedures to populations that are not morbidly obese. Bypassing of the foregut appears to be critical, perhaps because it tempers the release of hormonally active peptides from the gastrointestinal tract.33 In any case, the gut is regaining recognition as a major metabolic organ.
In light of these hypotheses, the duodenal-jejunal bypass is a bariatric procedure that may be beneficial for a patient with type 2 diabetes who is not morbidly obese. In this operation, the stomach volume is preserved but the foregut is bypassed. In a small experimental series from Brazil, patients with type 2 diabetes who were normal weight or only slightly overweight had resolution of their diabetes following this procedure, without any weight loss.34
New applications for endoscopy
Another area of development is endoluminal and transgastric bariatric surgery. Endoluminal surgery is performed entirely within the lumen of the gastrointestinal tract using flexible endoscopy. Transgastric surgery is performed within the peritoneal cavity, which is accessed via a hollow viscus. Both approaches use natural orifices to gain surgical access, thereby avoiding access incisions and scars.35
The benefits of such an approach are numerous:
(1) fewer complications and side effects; (2) less invasiveness, and thus the ability to perform in the outpatient setting; (3) reduced procedure costs; and (4) better access to treatment. The implication in terms of indications is the potential to use such procedures to prevent progression to morbid obesity.
Examples of these procedures are proliferating:
Gastrojejunostomy reduction is an endoscopic procedure that involves reducing the dilated opening of the gastric pouch after gastric bypass surgery. New endoscopic suturing or stapling devices enable the outlet reduction without requiring surgery. The result is enhancement of weight loss without a major operation.
Endoluminal suturing uses endoscopic instruments to suture the stomach to reduce its volume. When this procedure is perfected, the patient should be able to leave the endoscopy suite and return home within a few hours.
The duodenal sleeve is an avant garde concept in which an internal sleeve is threaded into the stomach and down the intestines.36 The sleeve covers the absorptive surface of the small bowel, preventing absorption of nutrients to cause weight loss. This procedure has been shown to have a strong antidiabetic effect as well.
Clinical applications of these operations are emerging. An endoluminal sutured gastroplasty procedure to shrink stomach volume has been shown in a small clinical trial to cause loss of significant excess body weight; the operation leaves no scars and is associated with a low risk of bleeding or any type of surgical complication.37 A similar procedure is in development that involves staples instead of sutures.
How best to validate innovations moving forward?
As we move into these new eras of metabolic surgery and endoluminal and transgastric bariatric surgery, interesting questions arise. We as innovators and caregivers are ethically obligated to demonstrate reasonable safety and efficacy before such new procedures are performed widely. Although some of these emerging procedures involve new devices that will go through the FDA review process, many are existing procedures for which indications may be expanded, while others are permutations of existing procedures for which no formal rules for validation exist. For new procedures that differ substantially from existing proven procedures but which do not require new devices, should we not be ethically bound to demonstrate safety and efficacy even though they do not require FDA review? These are the challenges that await as innovation takes bariatric surgery to new frontiers.
Natural orifice transluminal endoscopic surgery: Too much too soon?
By Christopher Thompson, MD, MHES
Although the endoscope has changed very little since the first fiberscope was developed 50 years ago, the accessories and other instruments used in conjunction with the endoscope have changed remarkably. These include clips for hemostasis, ultrasonographic technology, and instruments for tissue dissection.
These advances in endoscopy, combined with advances in laparoscopic surgery, have led to the convergence of these two fields, culminating in the new field of natural orifice transluminal endoscopic surgery (NOTES). In NOTES, the surgeon enters a natural orifice and punctures through a viscus to perform surgery, removes the endoscope, and closes the area without leaving a scar.
HISTORY OF NOTES AT A GLANCE
NOTES was patented as a concept in 1992. Its first application was as an exploratory procedure in the pig in 2004.38 Soon thereafter, therapeutic NOTES procedures in animals were reported, including tubal ligation, organ resection, cholecystectomy, and splenectomy.
Particularly notable in the development of NOTES is the extremely short interval between early animal experiments (2004) and the first human procedures, which took place as early as 2005 when surgeons in India used the technique to perform a human appendectomy. Since then, more than 300 NOTES procedures have been performed in humans throughout the United States, Europe, Latin America, and Asia, for applications ranging from percutaneous endoscopic gastrostomy rescue to transvaginal cholecystectomy.
This rapid adoption of NOTES in humans is concerning, as it raises clear questions about whether there has been time for adequate oversight and safety assessment. For instance, at a surgical conference in April 2008, questions and debate swirled around whether a large Brazilian registry of more than 200 NOTES cases did or did not include two deaths. Other ethical issues raised by NOTES are discussed further below.
DRIVING FORCES BEHIND NOTES
The medical rationale
Abdominal wounds can cause pain, are unaesthetic, and are prone to wound infections, ruptures, and hernias. They sometimes cause adhesions or may lead to abdominal wall syndromes with scar neuromas that cause pain later. They also require general anesthesia. Beyond these shortcomings of incision-based procedures, NOTES offers potential reductions in length of stay and therefore in cost. Moreover, certain patient populations may specifically stand to benefit from NOTES, such as obese patients, those with abdominal mesh in place, and those undergoing palliative procedures. This is the essence of the medical rationale for NOTES, which is somewhat thin.
Professional organizations and courses
In July 2005, leaders from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons convened a working group to support and plan for the responsible development of NOTES.39 The group formed the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR), an organization that has since sponsored several conferences on NOTES and procured millions of dollars in grants for NOTES research in animals. (In the interest of full disclosure, I am one of the founding members of NOSCAR.)
Additionally, leading institutions in this field have held numerous hands-on courses on NOTES throughout the United States, Europe, Latin America, and Asia. These courses, including those held by my laboratory at Harvard University, are designed to teach colleagues at other institutions how to set up an appropriate animal laboratory and to promote and encourage proper research in NOTES. There have been unintended consequences, however, as we have learned that some course attendees have returned to their home countries and immediately started using the techniques in humans.
New technology
At the July 2005 working group meeting that launched NOSCAR, we determined that several technological advances were needed before NOTES could be safely applied to humans. These included development of multitasking platforms, better devices for tissue apposition and fixation, better imaging and spatial orientation, and improved means of retraction.39 Industry responded with novel devices and end effectors such as guide tubes, direct drive systems, endoscopic suturing devices, magnetic retraction, devices for closing luminal defects, flexible staplers, and computerized robotics.
Other driving forces
Additional forces have undoubtedly contributed to the rapid development of NOTES:
- The slowdown in innovation in general surgery in recent years has left a vacuum to be filled.
- An abundance of venture capital has been available to rush into that vacuum.
- Perceived patient demand (owing to cosmetic advantages) has been a driver, especially in cities such as Rio de Janeiro, Milan, and New York.
- The fear of being left behind is a factor that cannot be underestimated. Surgeons who failed to convert to laparoscopic techniques from open techniques in the early 1990s for procedures such as cholecystectomy, fundoplication, and splenectomy were losing their patient bases. Many surgeons fear a similar phenomenon today if they do not adopt NOTES into their practices.
ETHICAL ISSUES RAISED BY NOTES
As NOTES moves toward further evaluation in humans, several ethical questions need to be grappled with:
- Must there be a significant potential for improvement in care before an innovation advances to human research?
- Is the cosmetic benefit of NOTES sufficient, considering the substantially increased risk? For instance, laparoscopic cholecystectomy is well established, whereas NOTES cholecystectomy carries an increased risk of bile duct injuries and other injuries. Is NOTES worth the risk?
- What about the corporate agenda behind new technologies and its associated influence on the media?
- Are hospital IRBs adequate to the task of evaluating and monitoring these questions, and will they be independent of the impact of hospitals’ larger agendas?
Finally, the problem of premature adoption of this technology is particularly concerning. I heard a surgeon explain at a course that he performed NOTES on a few pigs at a previous course and then returned home to Peru and immediately started performing it on patients at his ambulatory surgery center. There is also the temptation for well-respected surgeons to go to other countries to practice their NOTES skills before returning to the United States, in hopes that their experience will help them attain IRB approval. Practices like these raise questions about what ethical responsibilities lie with those of us who have pioneered the technology and are trying to develop and disseminate it responsibly. We can try to vigilantly watch course attendees from certain countries, but there is little we can do in the absence of regulation and enforcement in those countries. These are difficult ethical challenges.
Panel discussion
Moderated by Jonathan D. Moreno, PhD
Dr. Jonathan Moreno: I would like to begin with any questions that the panelists have for one another.
Dr. Philip Schauer: I would be curious to hear how my colleagues define incremental changes in a procedure. In other words, what constitutes a new procedure versus a modification of an existing one?
In bariatric surgery we are grappling with a procedure called the sleeve gastrectomy, which poses challenges comparable to lung volume reduction surgery as described by Dr. Cooper. Many of us believe that this procedure is just a slight modification of a gastroplasty, yet payors consider it an entirely different procedure, and some want 5 to 10 years of follow-up data before they will pay for the operation.
Dr. Joel Cooper: That is not an easy question, but I would approach it from the standpoint of what you would tell the patient. When we were first developing lung volume reduction, we performed it only in patients who had absolutely no other alternative. Only later in its development did we offer it as an alternative to transplantation. How do you approach the patient when you can already achieve a very good result with an existing procedure and you can tell the patient, with some assurance, what to expect with that procedure? In the case of NOTES, I do not think that the cosmetics are sufficient justification.
The second aspect is regulatory. I am not a supporter of the FDA’s practices for the introduction of new procedures, but I believe strongly that universities have been derelict in setting the standard for the introduction of new procedures, particularly minimally invasive procedures. They have been using these procedures as marketing tools to vie with private hospitals for dollars and patients. I cannot say whether the rapid promulgation of these procedures at too early a stage actually can be prevented, but I do not recall the chairmen of major surgery departments getting together to issue public statements about the proper protocol for introducing new techniques. As Pogo said, “We have met the enemy and he is us.”
This may not answer your question, but I believe there should be no payment for any new or novel procedure for a certain period after its introduction, and certainly the hospitals should not be able to profit from it, although the expenses of a new procedure may be recouped. That alone would perhaps put the brake on some of the marketing and the financial incentives, and it might separate, to some degree, the development of new procedures from economic interests.
WHO SHOULD OBTAIN INFORMED CONSENT?
Dr. Moreno: Should informed consent be obtained only by a knowledgeable third party rather than the surgeon-innovator?
Dr. Thomas Krummel: The question is whether there is a disinterested third party who truly is knowledgeable; in cases where there is such a person, I see no downside to having that person involved. However, the notion of having someone who is not associated with clinicians or surgeons obtaining informed consent makes me uncomfortable. Informed consent is not a piece of paper. It is a trust between physician and patient, and to ignore that could leave you in a heap of trouble.
Dr. Cooper: I agree, but another process is important as well. In proposing lung transplantation before there had been any successful transplants, we defined in advance the standards, indications, and contraindications that we thought should apply. We did this in the absence of any particular patient, and it relieved us of the difficulty of making arbitrary decisions that may have led to unfairly accommodating one patient over another. Once the standards have been set in this way, they can be applied—whether by the investigator or by a committee—in an objective way to the group of patients that is most appropriate in the early phases of development.
Dr. Ralph Clayman: It is difficult for the inventors of an operation to dampen their enthusiasm for their creation to a point where they are as objective as they should be. Joel is bringing up situations for which there are no alternatives. My realm is an area in which there were well-established alternatives for everything we have done laparoscopically or percutaneously, and it was difficult to decide the indications or contraindications early on. Often, the early indications only had to clear the threshold of not seeming ridiculous.
The early development of percutaneous stone removal at the University of Minnesota took place entirely outside the purview of an IRB. Percutaneous nephroscopy had been around since 1955, and we extended it to plucking out a stone. That is how that entire field developed. Early on, we were not going to go after a stone that was as big as a fist because we did not have a way to break it up. As time went by, however, it evolved to the point where there was no stone in the kidney—regardless of its size, location, or hardness— that could not be removed through a small hole in the back. But that entire evolution proceeded without IRB approval.
For laparoscopic nephrectomy, for which there were well-known alternatives, who should have obtained the informed consent? Should it have been me, bringing along the “white coat” factor and not being able to really explain the potential problems since nobody had yet gone there, despite my rapport with the patient? Or should it have been a third party with whom I had discussed the procedure and its possible problems? I do not know the answer, but it raises an interesting point, especially in this age of IRBs and ethics committees.
Dr. Krummel: It is not unlike what we have tried at Stanford when we are not sure of the boundary for IRB consultation. The surgical chairs are willing to convene and essentially police one another, so that when the neurosurgeon proposes a brain transplant, there probably will be a pretty interesting conversation before it gets the green light.
Dr. Cooper: My experiences with IRB involvement differed quite a bit between my work in lung transplantation and my work in volume reduction surgery, but the differences owe a lot to the countries where I was practicing at the time. I did my early work in transplantation in Canada, where I did ask for approval from my hospital’s ethics committee and other relevant committees. In Canada, the hospital had a global budget, and it made a decision that it was willing to use part of its budget for transplantation. We received no fees for years, until the operation was proven to be effective, but that did not stop us from developing the procedure.
I had returned to the United States when I began my work with lung volume reduction, and I did not ask the IRB for permission to do that procedure. My justification was that, theoretically, volume reduction was similar to accepted practices for removal of nonfunctioning lung to improve respiratory mechanics (bullectomy) and that we would simply be applying the concept to a different group of patients. However, unlike in Canada, I did not have institutional financial support for doing this new procedure, so how was I going to do it if the hospital could not receive payment for it? I went to the IRB, but instead of asking for permission to do the procedure I asked for permission to study the procedure and to collect data on it. In that way, I was notifying the IRB of my action and thus giving it an opportunity to act. If I had gone to the IRB to approve the procedure, however, the operation would have been labeled experimental by insurance companies, who would have then found a way to deny payment. At least that was how it was in those days.
MARKETING OF MEDICINE: IS THERE NO TURNING BACK?
Question from audience: What makes you think that in 10 years there won’t be 100 million obese Americans watching television ads for noninvasive bariatric surgery promising to rid them of their obesity problem? What will keep that from happening?
Dr. Krummel: Nothing. What makes you think it is not happening now? Just look at the ads for the Lap-Band in the lay press.
Dr. Clayman: We already have direct marketing of drugs and direct marketing of facilities. What Joel said is true: “the enemy is us.” When I was in training, the idea that a physician would advertise was considered unethical. I still consider it thus. But everybody is doing it, so should that make it acceptable? I think not.
The same thing is true of the huge amount of money spent marketing drugs on television. Why should a single nickel be spent to advertise health care beyond generically informing the public of important health care issues and initiatives? You cannot go to an airport without seeing a surgical robotics program being advertised or a hospital being advertised. You cannot turn on National Public Radio without hearing well-financed spots touting the achievements of a hospital. You cannot watch television without seeing ads for erectile dysfunction medications or other new drugs. It is a waste of dollars. If we took all of that money and redirected it, we could probably solve much of the indigent health care problem, but we as a society have chosen not to do that.
SHOULD THE BAR BE RAISED FOR SURGICAL TRIALS?
Dr. Moreno: Let’s consider some additional questions. Why shouldn’t the government raise the bar on the level of evidence needed to gain regulatory approval for new devices? Why not require randomized trials, as is done for drugs?
Dr. Cooper: Procedures that lend themselves to a randomized trial should be studied at a limited number of centers with mandatory reporting and preset indications for promulgation and payment. I believe that universities have been derelict in their duty to require this level of evidence.
This question is always nuanced, however. Consider the case of laparoscopic procedures. They offer the advantage of smaller incisions, yet how many patients have had to die or suffer serious consequences for the sake of these smaller incisions? On the other hand, how many patients may have been saved from pulmonary embolism, wound infections, or a prolonged hospital stay as a result of laparoscopic techniques? Only a randomized trial could demonstrate whether or not there has been an overall payback from new procedures such as this, although even then the payback may be present for some types of patients but not others.
Dr. Schauer: The problem is expense. Perhaps it is all a matter of economics. Return on investment for the drug industry is something like 10 to 1, but return on investment for the medical device industry is generally much lower. Therefore, conducting large randomized controlled trials is extremely expensive and much more complicated for a device or procedure. This may explain why the standard for trials is different for the two industries.
Dr. Krummel: Virtually all fetal surgical procedures have been subjected to a trial, several of them randomized. The National Institutes of Health paid for many of these trials. One such study prevented rapid uptake of the congenital diaphragmatic hernia operation, which has never been proven in a randomized trial to be better than our current therapy. It is a good example of a randomized trial making a difference.
Dr. Clayman: As Joel pointed out, surgery is constantly evolving, whereas a drug remains unchanged throughout its lifespan. If we had started a prospective randomized trial after we had done our first laparoscopic nephrectomy, the procedure would have died because we were not nearly as facile with our first 10 as we were after our first 100. The technology continues to develop, and the surgeon continues to develop his or her skills, which makes a study of this nature overly dynamic. Perhaps the best you can do is a retrospective, matched, controlled study with the same surgeon, comparing his or her results after 40 or 50 laparoscopic procedures with results after his or her 50 most recent open procedures.
Dr. Cooper: How do you put a brake on the system? Would some sort of limited trial perhaps put a brake on the too-rapid promulgation that we often see?
Dr. Clayman: In the general surgery realm, laparoscopic cholecystectomy came out of private practice. It did not come out of the university with its faculty and laboratories dedicated to exploration and investigation. It never was properly vetted in the scientific realm but rather came to the light of day as an “economic” edge.
Dr. Krummel: I would not underestimate the talent and creativity of those that we train who go out into private practice. Much innovation has come from very active practitioners.
Dr. Clayman: Right, but they do not have the infrastructure that we are blessed with at universities both to create and to validate.
Dr. Schauer: I agree that academia does not have a monopoly on creative ideas. But perhaps academia should play a major role in defining validation-type studies. That is one area where we may be especially well suited to meet an important need.
THE INNOVATION-TRAINING INTERFACE
Question from audience: I have a dilemma as a residency program director. Our residents want to learn the new technology—laparoscopic surgery, robotic surgery, etc—but we have them in our program for such a limited time. How do you justify teaching them new technology and at the same time still teach them basic, traditional surgical procedures, especially with the reduction in residents’ hours? It is fine to be able to do a nephrectomy laparoscopically, but if you get in trouble, you still have to know how to do a good open nephrectomy. How do you address this?
Dr. Schauer: I think the answer largely is fellowship training. Emerging procedures probably should be introduced in fellowship programs until they reach the point where they are so standardized that they become a major part of practice. For example, cholecystectomy quickly became part of general surgery practice, but laparoscopic colectomy took several years to evolve and was taught primarily in fellowship or advanced training, after which it gradually filtered down to residency programs.
Dr. Krummel: All of us who are responsible for training are wrestling with this problem. Residents are expected to learn more yet do so in less time. One approach would be early specialization, so that instead of 5 years of general surgery, you would have 3 years of general surgery and then 3 years of, for instance, thoracic surgery. Also, Ralph mentioned earlier the advantage of skills labs. We increasingly see that type of approach as a backbone for providing broad training without putting patients at harm.
As for teaching the use of new technology, first you have to teach the existing base of practicing surgeons. Here again there is much to be said for skills labs, and I give credit to the American College of Surgeons for its drive to establish and accredit centers around the country as a way to teach this base of surgeons.
Dr. Schauer: If I may expand this question beyond residency and fellowship training, how do we balance the desire to share new innovations with our colleagues against the need to temper their desire to prematurely jump into an area where they do not yet really belong? Chris, I know this applies to your challenges in disseminating knowledge about NOTES.
Dr. Christopher Thompson: Yes, courses on NOTES are also being held in conjunction with all the major society meetings, and we are seeing many enthusiastic trainees at these hands-on courses. The original intent was to give attendees instruction on setting up their own animal labs, yet some trainees took it beyond this limited purpose. As a result, some in our field believe that we should not allow foreign physicians to come here to be trained in NOTES, for fear they will go back to their home countries and use it on humans. I am not certain that that approach is the best way to go, but there has been much discussion about how to handle this. It is a real conundrum. Certainly there are a number of surgical residents and gastroenterology fellows who are clamoring to get into the lab right now and learn these techniques.
Dr. Clayman: This goes back to our earlier discussion about from where new technologies should emerge. What frightens me are the consequences of creative activity occurring outside the university, where there are no laboratories or animal or cadaver models for refining or testing a technique. To me, it was frightening to see laparoscopic cholecystectomy suddenly emerge as a craze without the proper animal and clinical studies having been done. That is not the way I believe clinical research should go forward. I once heard a prominent urologic surgeon say at a major surgical meeting, after a presentation on the impact of percutaneous stone surgery on the canine kidney, “Now that I’ve done a thousand of these in humans, it’s reassuring to know that it’s safe to do in dogs.” That is not the way it should happen, and every time it does happen that way, we pay a large price, some of us as individuals and all of us as a society.
Dr. Cooper: The answer therefore is to use our academic facilities to facilitate the training of those in community practice. We should continue to offer training because we have the resources to make it available.
Dr. Clayman: Yes, and this is why I emphasized earlier that support for surgical training centers is so essential. I see all the dollars spent on health care advertising and wonder why these dollars are not instead poured into surgical training, or research facilities, or training simulators.
The way we should train surgeons in new technologies is to train them on simulators equipped with a properly vetted curriculum. This is the future for training, because once you put instruments through small ports, everything becomes measurable—economy of motion, past pointing, and efficiency; simulators with a curriculum will also be able to assess the trainee’s cognitive abilities. When an individual performs well on the simulator, he or she can then come into the operating room and work with surgeons experienced in the procedure. The use of simulators in this manner should ultimately improve the overall quality and safety of each surgical specialty.
RISE OF THE ROBOTS
Question from audience: I am curious how the panel members interpret early randomized trial data showing an increased cost without an improvement in care with the use of surgical robots in certain procedures. Should we persist or consider an investment in the future as robotic technology improves and surgeons further adapt to it?
Dr. Cooper: I think the robot should be used only for those procedures for which it has unique capability and can perform a task better than we can. It appears that the robot performs better than the ear, nose, and throat surgeon for operations on the base of the tongue. The same may be true of prostate surgery, but I am not certain. But to do a laparoscopic Nissen repair with a robot…as Dr. Nat Soper of Northwestern University has said, “If I needed a robot, I shouldn’t be doing laparoscopic Nissens.”
The robot provides light, it gives you magnification, and it reduces tremor. We should concentrate on its use for operations where these attributes are particularly valuable. But we should be wary of its use as an expensive marketing tool.
Dr. Clayman: The robot provides you with superhuman capabilities: 10 to 30X magnification, no tremor, a 540-degree wrist, instrumentation with 6 degrees of freedom, and motion scaling. It allows you to be a better surgeon than you are without it. I agree that it is expensive. It is woefully overpriced at this point, but I believe the expense will come down with time. It is no different than the first computers, which were terribly expensive. The robot enables surgeons to do a better job than they would without it if we are talking about reconstructive-type surgery.
Ergonomically, the robot is very positive for the surgeon. For the first time, the surgeon is actually allowed to sit down in a comfortable environment and can work for 4 hours straight, get up at the end of the surgery, and feel fine. If you are older than 50 and you operate standing at the table staring at a television screen on the other side for 4 to 6 hours, you are going to ache afterwards. I believe surgeons work better if they are comfortable.
Dr. Schauer: At least within my field of general surgery, there has been no evidence that this superhuman ability has translated into superhuman results, in terms of reduced operating time, fewer complications, or better efficacy. We should probably develop the metrics to measure progress. How do the theoretical benefits translate into clinical benefit?
Dr. Clayman: It is not theoretical in radical prostatectomy if you look at the data. The potency rates for patients who undergo robotic surgery for these procedures are now almost 90%, which is something that no surgeon performing open prostatectomy has ever achieved. Fortunately, the continence mechanism is so strong in most adults that it does not matter whether prostatectomy is done with a robot or open surgery—patients are probably going to be all right. But the bottom line is that robotic surgery is a bit better. Most surgeons would use it if it were free. The problem is that it is so expensive right now and it is breaking the backs of many hospitals.
Dr. Schauer: You make a good point. Demonstrating metrics is important, and prostatectomy is a good example. But I am not aware of any other procedures for which benefit from robotic surgery has been documented.
Dr. Krummel: The history of robotic surgery is so interesting because the killer application was supposed to be coronary work—percutaneous bypass surgery. But then the heart port went to pot and patients with anterior wall lesions ended up not being a big enough group. It turns out that it is still difficult to do and there is not a lot of room. So prostatectomy has ended up as the initial killer application.
Keep in mind that the current robot is not an end device. We will see more. There are now robotic steerable catheters that I think will be adopted into NOTES procedures. This theme of immediate benefit versus follow-on iterations is the story of device development in this country.
- Gorman C. Are surgeons too creative? Time 1995; 146(Sept 4):56–57.
- Majeed AW, Troy G, Nicholl JP, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet 1996; 347:989–994.
- Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996; 347:984–985.
- Reeves B. Health-technology assessment in surgery. Lancet 1999; 353(suppl 1):3–5.
- Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Allen KB, Dowling RD, Fudge TL, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med 1999; 341:1029–1036.
- The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999; 116:1750–1761.
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345:1075–1083.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med 1986; 28:746–750.
- Berger RL, Celli BR, Meneghetti AL, et al. Limitations of randomized clinical trials for evaluating emerging operations: the case of lung volume reduction surgery. Ann Thorac Surg 2001; 72:649–657.
- Clayman RV, Kavoussi LR, Long SR, Dierks SM, Meretyk S, Soper NJ. Laparoscopic nephrectomy: initial report of pelviscopic organ ablation in the pig. J Endourol 1990; 4:247–252.
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991; 146:278–282.
- Colegrove PM, Winfield HN, Donovan JF Jr, See WA. Laparoscopic practice patterns among North American urologists 5 years after formal training. J Urol 1999; 161:881–886.
- Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2(5402):177.
- Beecher HK. Ethics and clinical research. N Engl J Med 1966; 274:1354–1360.
- The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: Department of Health, Education, and Welfare; April 18, 1979. Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.htm. Accessed July 28, 2008.
- Moore FD. Three ethical revolutions: ancient assumptions remodeled under pressure of transplantation. Transplant Proc 1988; 20(suppl 1):1061–1067.
- Moore FD. The desperate case: CARE (costs, applicability, research, ethics). JAMA 1989; 261:1483–1484.
- MacDonald KG Jr, Long SD, Swansons MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997; 1:213–220.
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004; 199:543–551.
- Christou NV, Sampalis JS, Lieberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004; 240:416–423.
- Sowemimo OA, Yood SM, Courtney J, et al. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis 2007; 3:73–77.
- O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006; 16:1032–1040.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222:339–350.
- Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 2005; 9:1112–1116.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003; 237:751–756.
- Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis 2006; 2:401–404.
- Dixon JB, Dixon AF, O’Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese: homeostatic model assessment. Diabet Med 2003; 20:127–134.
- Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res 2004; 36:735–741.
- Wajchenberg B, Cohen R, Schiavon C, et al. Laparoscopic duodenal jejunal bypass in the treatment of type 2 diabetes mellitus in patients with BMI < 34. Initial results. Abstract presented at: 68th Annual Scientific Sessions of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 1454-P.
- Schauer P, Chand B, Brethauer S. New applications for endoscopy: the emerging field of endoluminal and transgastric bariatric surgery. Surg Endosc 2007; 21:347–356.
- Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244:741–749.
- Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 2008; 68:51–58.
- Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc 2004; 60:114–117.
- Rattner D, Kalloo A, SAGES/ASGE Working Group on Natural Orifice Translumenal Endoscopic Surgery. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. Surg Endosc 2006; 20:329–333.
- Gorman C. Are surgeons too creative? Time 1995; 146(Sept 4):56–57.
- Majeed AW, Troy G, Nicholl JP, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet 1996; 347:989–994.
- Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996; 347:984–985.
- Reeves B. Health-technology assessment in surgery. Lancet 1999; 353(suppl 1):3–5.
- Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Allen KB, Dowling RD, Fudge TL, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med 1999; 341:1029–1036.
- The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999; 116:1750–1761.
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345:1075–1083.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med 1986; 28:746–750.
- Berger RL, Celli BR, Meneghetti AL, et al. Limitations of randomized clinical trials for evaluating emerging operations: the case of lung volume reduction surgery. Ann Thorac Surg 2001; 72:649–657.
- Clayman RV, Kavoussi LR, Long SR, Dierks SM, Meretyk S, Soper NJ. Laparoscopic nephrectomy: initial report of pelviscopic organ ablation in the pig. J Endourol 1990; 4:247–252.
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991; 146:278–282.
- Colegrove PM, Winfield HN, Donovan JF Jr, See WA. Laparoscopic practice patterns among North American urologists 5 years after formal training. J Urol 1999; 161:881–886.
- Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2(5402):177.
- Beecher HK. Ethics and clinical research. N Engl J Med 1966; 274:1354–1360.
- The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: Department of Health, Education, and Welfare; April 18, 1979. Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.htm. Accessed July 28, 2008.
- Moore FD. Three ethical revolutions: ancient assumptions remodeled under pressure of transplantation. Transplant Proc 1988; 20(suppl 1):1061–1067.
- Moore FD. The desperate case: CARE (costs, applicability, research, ethics). JAMA 1989; 261:1483–1484.
- MacDonald KG Jr, Long SD, Swansons MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997; 1:213–220.
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004; 199:543–551.
- Christou NV, Sampalis JS, Lieberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004; 240:416–423.
- Sowemimo OA, Yood SM, Courtney J, et al. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis 2007; 3:73–77.
- O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006; 16:1032–1040.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761.
- Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752.
- Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222:339–350.
- Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 2005; 9:1112–1116.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238:467–484.
- Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003; 237:751–756.
- Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis 2006; 2:401–404.
- Dixon JB, Dixon AF, O’Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese: homeostatic model assessment. Diabet Med 2003; 20:127–134.
- Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res 2004; 36:735–741.
- Wajchenberg B, Cohen R, Schiavon C, et al. Laparoscopic duodenal jejunal bypass in the treatment of type 2 diabetes mellitus in patients with BMI < 34. Initial results. Abstract presented at: 68th Annual Scientific Sessions of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 1454-P.
- Schauer P, Chand B, Brethauer S. New applications for endoscopy: the emerging field of endoluminal and transgastric bariatric surgery. Surg Endosc 2007; 21:347–356.
- Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244:741–749.
- Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 2008; 68:51–58.
- Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc 2004; 60:114–117.
- Rattner D, Kalloo A, SAGES/ASGE Working Group on Natural Orifice Translumenal Endoscopic Surgery. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. Surg Endosc 2006; 20:329–333.