User login
Convertible Glenoid Components Facilitate Revisions to Reverse Shoulder Arthroplasty Easier: Retrospective Review of 13 Cases
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.
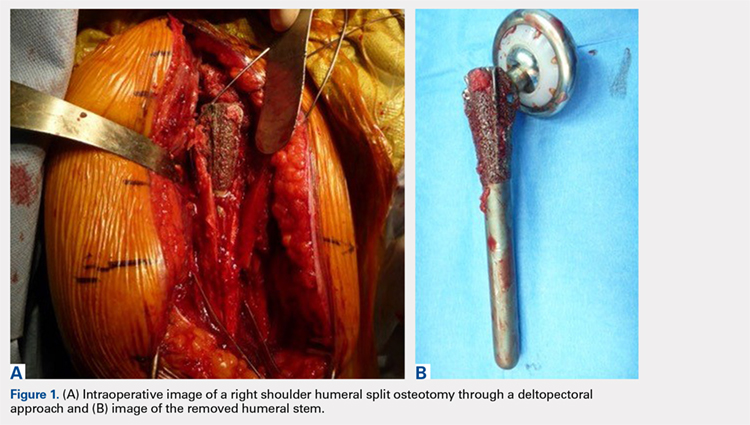
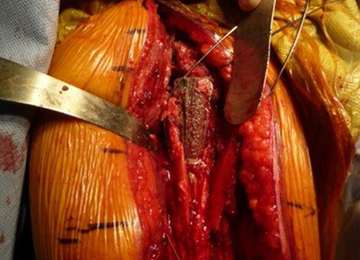
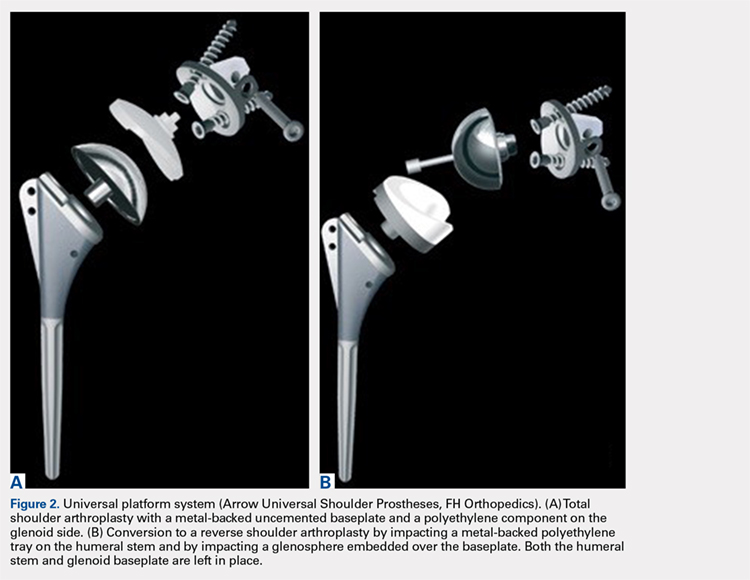
We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).
The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
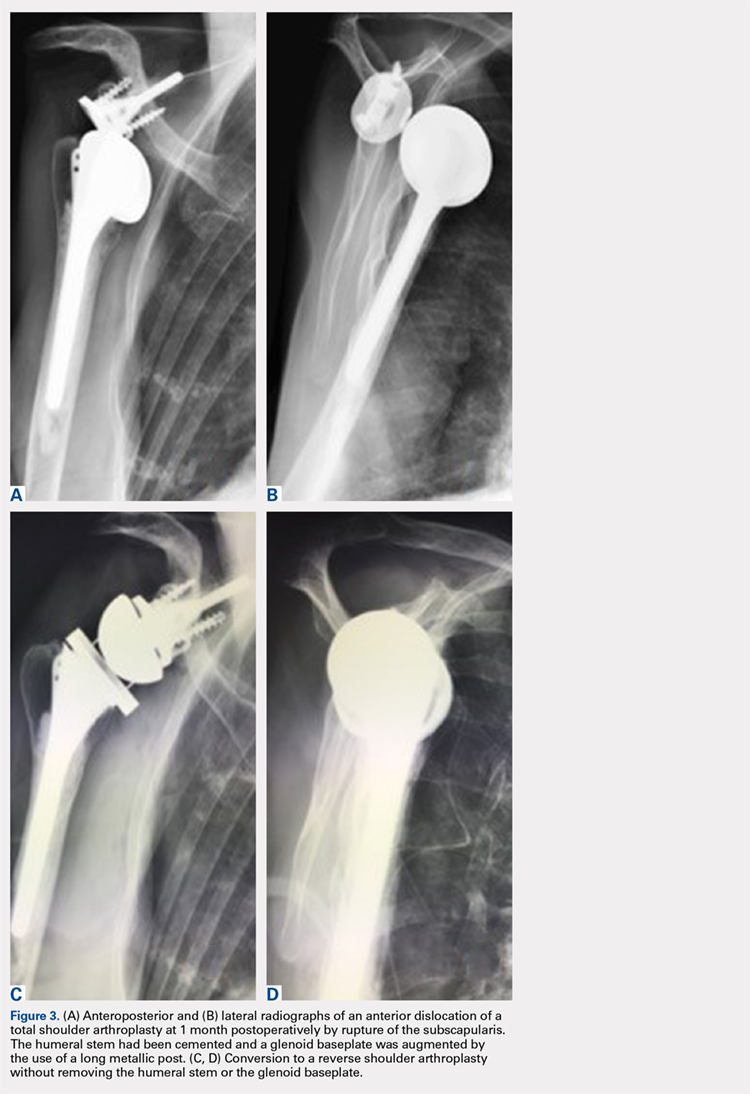
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.
In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.

We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).

The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.

In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.

We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).

The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.

In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
TAKE-HOME POINTS
- Full polyethylene is the gold standard, but the revision of glenoid loosening leads a difficult reconstruction of a glenoid bone.
- A complete convertible system facilitates the revision and decreases the rate of complications.
- The functional and subjective results of the revision are good.
- During the revision, the metalback was well fixed without any sign of loosening.
- In 3 cases the humeral stem was changed; in 2 cases there was no space to reduce (onlay system) and in 1 case it was an older design, nonadapted.