User login
Changes to practice may help avoid ‘double trouble’
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
Airway pressure release ventilation: An alternative mode of mechanical ventilation in acute respiratory distress syndrome
In the early stages of acute respiratory distress syndrome (ARDS), multiple areas of the lung collapse, most often in the dependent regions. A factor involved in this process is the loss of functional surfactant, creating a condition in which alveolar units are unstable and prone to collapse due to unopposed surface tension. This situation, similar to that in premature infants, results in a reduced volume of aerated lung, intrapulmonary shunting, and, therefore, poor oxygenation.
The treatment of this alveolar collapse is lung reinflation (or “recruitment,” a term first used by Lachmann).1 Gattinoni et al2 showed that the percentage of recruitable lung could range from a negligible fraction to 50% or more.
There are various means of reopening injured lungs and keeping them open. The choice of recruitment maneuver is based on the individual patient and the ventilatory mode.3
In this article, we review airway pressure release ventilation (APRV), a mode of mechanical ventilation that may be useful in situations in which, due to ARDS, the lungs need to be recruited and held open. APRV was developed as a lung-protective mode, allowing recruitment while minimizing ventilator-induced lung injury.
BASIC PRINCIPLES OF PROTECTIVE VENTILATION
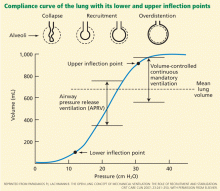
This curve has two inflection points between which its slope is steep, indicating greater compliance or elasticity. Below the lower inflection point, the alveoli may collapse; above the upper inflection point, the lung loses its elastic properties and the alveoli are overdistended. To protect the lungs, the challenge in mechanical ventilation is to keep the lungs between these two points throughout the respiratory cycle.
Avoiding lung collapse by using PEEP
During mechanical ventilation, the pressure in the lungs is lowest, and thus the alveoli are most prone to collapse, at the end of expiration.
We want to prevent the alveoli from collapsing with each expiration and reopening with each inspiration, as this cycle of opening and closing damages them (causing atelectrauma, ie, cyclical atelectasis).4 Preventing it prevents the release of inflammatory mediators and the perpetuation of lung injury (biotrauma).5
The solution is to apply positive end-expiratory pressure (PEEP), taking into account the value of the lower inflection point when setting the PEEP level.
Villar et al6 compared outcomes in an intervention group that received a PEEP level 2 cm H2O above the lower inflection point plus low tidal volumes, and in a control group that received higher tidal volumes and low PEEP (5 cm H2O). The study was stopped early, after significantly more patients had died in the control group than in the intervention group (53% vs 32%, P = .04).
Avoiding overdistention by keeping the tidal volume low
Tidal volumes that exceed the upper inflection point overstretch the lung and induce volutrauma, which can manifest as pneumothorax or pneumomediastinum, or both—the lungs rupture like a balloon. Also, overdistention produces liberation of inflammatory mediators in the blood (biotrauma). High tidal volumes should therefore be avoided or limited as much as possible.
The ARDS Network,7 in a multicenter, randomized, controlled trial, showed that fewer patients die if they receive mechanical ventilation with low tidal volumes rather than higher, “conventional” tidal volumes. Patients were randomized to receive either a tidal volume of 6 mL/kg and a plateau pressure lower than 30 cm H2O or a tidal volume of 12 mL/kg and a plateau pressure lower than 50 cm H2O. They were followed for 180 days or until discharged home, breathing without assistance. A total of 861 patients were enrolled. The mortality rate was significantly lower in the low tidal volume group than in the group with conventional tidal volumes, 31% vs 40%.
Lower tidal volumes were also associated with faster attenuation of the inflammatory response.8
Amato et al9 randomized 58 patients to receive mechanical ventilation with tidal volumes of either 6 mL/kg or 12 mL/kg. The PEEP level was maintained above the lower inflection point. At 28 days, 62% of the patients in the intervention group were still alive, compared with only 29% in the control group. However, many concerns were expressed over the high mortality rate in the control group.
Based on these studies, the use of low tidal volumes with appropriate levels of PEEP to ensure lung recruitment is the current standard of care in mechanical ventilation of patients with ARDS.10
APRV: A PRESSURE-CONTROLLED MODE THAT ALLOWS SPONTANEOUS BREATHS
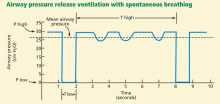
A baseline high pressure (P high) is set first. Mandatory breaths are achieved by releasing the high baseline pressure in the circuit very briefly, usually to 0 cm H2O (P low), which allows the lungs to partially deflate, and then quickly resuming the high pressure before the unstable alveoli can collapse.
In theory, the optimal release time (the very short time in low pressure, or T low) in APRV should be determined by the time constant of the expiratory flow. The time constant (t) is the time it takes to empty 63% of the lung volume. It is calculated as:
t = C × R
where C is the combined compliance of the lung and chest wall, and R is the combined resistance of the endotracheal tube and the natural airways. In diseases that lead to lower lung compliance (such as ARDS), the time constant is shorter. A practical equilibrium time—or the time it takes for the lung volume in expiration to reach steady state (no expiratory flow)—is about 4 time constants.14
Since the release time in APRV is much shorter than the equilibrium time, a residual volume of air remains in the lung, creating intentional auto-PEEP. Ideally, this intentional auto-PEEP should be high enough to avoid derecruitment (optimally above the lower inflection point). In APRV the auto-PEEP is controlled by the settings, and this intentional restriction of the expiratory flow is critical to avoid derecruitment of unstable alveolar units.
The amount of time spent at the higher pressure (T high) is generally 80% to 95% of the cycle (ie, the lungs are “inflated” 80% to 95% of the time), and the amount of time at the lower pressure (T low) is 0.6 to 0.8 seconds.
Thus, APRV settings provide a relatively high mean airway pressure, which prevents collapse of unstable alveoli and over time recruits additional alveolar units in the injured lung. The major difference between this mode and more conventional modes is that in APRV the mean inspiratory pressure is maximized and end-expiratory pressure is due to intentional auto-PEEP. In addition, spontaneous breathing is allowed throughout the entire cycle (Figure 2).13
Although APRV does not approximate the physiology of spontaneous breathing with healthy lungs, it is nonetheless relatively comfortable and well tolerated. Its theoretical advantage in patients with lung injury is its ability to maximize alveoli recruitment by maintaining a higher mean inspiratory pressure, while the peak alveolar pressure remains lower than with conventional ventilation (Figure 1).
Other modes that are similar to APRV
Other modes of mechanical ventilation very similar to APRV are biphasic positive airway pressure (BiPAP) and bilevel ventilation.
BiPAP differs from APRV only in the timing of the upper and lower pressure levels. In BiPAP, T high is usually shorter than T low. Therefore, in order to avoid derecruitment, P low has to be set above zero with both a high and a low PEEP level.13
No studies have demonstrated one mode to be more beneficial than the other, although BiPAP might be more predictable, as both pressures are known.
Bilevel ventilation works like APRV but incorporates pressure support to spontaneous breathing. The use of pressure support may affect the positive physiologic effects (see section below) of unsupported spontaneous breathing. Nevertheless, this strategy might be useful to address severe hypercapnia in the context of APRV.
INITIAL VENTILATOR SETTINGS IN APRV
P high. In selecting an initial P high, we measure the plateau pressure in a conventional mode using an accepted protective strategy, such as volume-control mode. If the plateau pressure is lower than 30 cm H2O, we use this pressure as our initial P high. If the plateau pressure is higher than 30 cm H2O, we select 30 cm H2O as an initial P high to minimize peak alveolar pressure and reduce the risk of lung overdistention.
P low is set at 0 cm H2O.
T high is set at 4 seconds and is then adjusted if necessary.
T low is probably the most difficult variable to set because it needs to be short enough to avoid derecruitment but still long enough to allow alveolar ventilation. We usually start with a T low of 0.6 to 0.8 seconds.
ADJUSTING THE VENTILATOR SETTINGS
For hypoxemia. Physician-controlled variables that affect oxygenation in APRV are:
- Mean airway pressure (dependent primarily on P high and T high)
- Fraction of inspired oxygen (Fio2).
Inadequate oxygenation usually requires increasing one or both of these settings.
Physician-controlled variables that affect alveolar ventilation in the APRV mode are:
- Pressure gradient (P high minus P low)
- Airway pressure release time (T low)
- Airway pressure release frequency.14 Frequency is related to total cycle time of mandatory breaths by the following equation3:
frequency = 60/cycle time = 60/(T high + T low).
Note that if T low remains constant, adjusting T high will adjust frequency (the more time the lung remains inflated, the lower the respiratory frequency). Conversely, some ventilators allow adjustment of frequency, making T high the dependent variable. The goal of this mode is to recruit alveoli and improve oxygenation, so we usually do not modify the pressure gradient to improve ventilation.
For hypercapnia. A frequent and expected consequence of lung-protective ventilation strategies is hypercapnia, termed “permissive” hypercapnia because it is allowed to some extent. In APRV, some degree of CO2 retention is not unusual. When the measured Paco2 becomes extreme, we usually increase the frequency of releases by shortening T high, recognizing that this adjustment may affect recruitment by lowering the mean airway pressure.
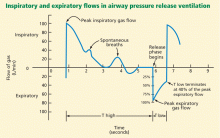
Spontaneous breaths. A positive aspect of APRV that contributes to its tolerability for patients is that it allows for spontaneous respiration. In some studies of patients with ARDS ventilated with APRV, spontaneous breathing accounted for 10% to 30% of the total minute ventilation and was responsible for an improvement in ventilation-perfusion matching and oxygenation.15,16 We titrate our patients’ sedation to a goal of spontaneous breathing of at least 10% of total minute ventilation.
WEANING FROM APRV
Weaning from APRV is done carefully to avoid derecruitment. Some authors recommend lowering P high by 2 to 3 cm H2O at a time and lengthening T high by increments of 0.5 to 2.0 seconds.13,17
Once P high is about 16 cm H2O, T high is at 12 to 15 seconds, and spontaneous respiration accounts for most or all of the minute volume, the mode can be changed to continuous positive airway pressure (CPAP) and titrated downwards. Usually, when CPAP is at 5 to 10 cm H2O, the patient is extubated, provided that mental status or concerns about airway protection or secretions are not contraindications.
PHYSIOLOGIC EFFECTS OF APRV WITH SPONTANEOUS BREATHING
Effects on the respiratory system
During spontaneous breathing, the greatest displacement of the diaphragm is in dependent regions. These regions are the best ventilated.18 Compared with spontaneously breathing patients, mechanically ventilated patients have a smaller inspiratory displacement of the dependent part of the lung.19
A study using computed tomography demonstrated that the reduction of lung volume observed in patients with acute lung injury (ALI) predominantly affects the lower lobes (dependent areas).20 Causative mechanisms could be an increase in lung weight related to ALI and a passive collapse of the lower lobes associated with an upward shift of the diaphragm.
In a preliminary study, the topographic distribution of lung collapse was different in spontaneously breathing ARDS patients than in patients who were paralyzed. In particular, lung densities were not concentrated in the dependent regions in the former group.21
Oxygenation is better with APRV with spontaneous breathing than with mechanical ventilation alone. This effect is at least partly attributable to recruitment of collapsed lung tissue and increased aeration of the dependent areas of the lung.22
Putensen et al15 compared ventilation-perfusion distribution in 24 patients with ARDS who were randomized to APRV with spontaneous breathing (more than 10% of the total minute ventilation), APRV without spontaneous breathing, or pressure-support ventilation. Spontaneous breathing during APRV improved ventilation-perfusion matching and increased systemic blood flow.
Neumann et al23 recently compared the effect of APRV with spontaneous breathing vs APRV without spontaneous breathing in terms of ventilation perfusion in an animal model of lung injury. APRV with spontaneous breathing increased ventilation in juxta-diaphragmatic regions, predominantly in dependent areas. Spontaneous breathing had a significant effect on the spatial distribution of ventilation and pulmonary perfusion.
Based on these studies, we generally use APRV with no pressure support. This strategy permits recruitment and expansion of dependent lung areas.
Effects on the cardiovascular system and hemodynamics
Räsänen et al,24 in an animal model, compared cardiovascular performance during APRV, spontaneous breathing, and continuous positive pressure ventilation. No significant differences in cardiovascular function were detected between APRV and spontaneous breathing. In contrast, continuous positive pressure ventilation decreased blood pressure, stroke volume, cardiac output, and oxygen delivery.
Falkenhain et al,25 in a subsequent case report, found that a change in mode from intermittent mandatory ventilation with PEEP to APRV resulted in improvement in the cardiac output of a patient requiring mechanical ventilation.
The lack of deleterious effect of APRV on cardiovascular function is probably a result of its spontaneous breathing component. The reduction in mean intrathoracic pressure during spontaneous breathing (compared to paralysis) improves venous return and biventricular filling, boosting cardiac output and oxygen delivery.26
Hering et al27 compared APRV with spontaneous breathing (at least 30% of the total minute ventilation) vs APRV with no spontaneous breathing in 12 patients with ALI. This study showed higher renal blood flow, glomerular filtration, and osmolar clearance in the APRV-with-spontaneous-breathing group.
The same investigators evaluated the effects of spontaneous breathing with APRV on intestinal blood flow in an animal model of lung injury.28 Spontaneous breathing with APRV improved arterial oxygenation, the systemic hemodynamic profile, and regional perfusion to the stomach and small bowel compared with full ventilatory support.
ANIMAL STUDIES OF APRV
Stock et al,11 in their original description of APRV in 1987, reported experimental results in dogs. In that study, 10 dogs with and without ARDS were randomized to APRV with a custom-built device vs volume-control mode with a Harvard pump ventilator plus PEEP. APRV delivered adequate alveolar ventilation, had lower peak airway pressures, and promoted better arterial oxygenation (at the same tidal volume and mean airway pressure) compared with volume control.
Martin et al (1991)29 studied seven neonatal lambs with ALI with four ventilatory modes: pressure-support ventilation, APRV, volume control, and spontaneous breathing. APRV maintained oxygenation while augmenting alveolar ventilation compared with pressure-support ventilation. APRV also provided ventilation at a lower peak pressure in contrast to volume control. The authors concluded that APRV was an effective mode to maintain oxygenation and assist alveolar ventilation with minimal cardiovascular impact in their animal model of ALI.
HUMAN STUDIES OF APRV
Garner et al (1988)30 studied 14 patients after operative coronary revascularization, giving them volume control mode (12 mL/kg) and then, when they were hemodynamically stable, APRV. While APRV and volume control supported ventilation and arterial oxygenation equally in all cases, peak airway pressure was greater with volume control.
Räsänen et al (1991)31 designed a prospective, multicenter, crossover trial in which 50 patients with ALI were ventilated with conventional ventilation and subsequently with APRV. Patients in both groups were adequately ventilated and oxygenated. However, as described in the aforementioned study,24 the peak airway pressure was lower in the APRV group.
Davis et al (1993)32 studied 15 patients with ARDS requiring ventilatory support who received intermittent mandatory ventilation plus PEEP and then were placed on APRV. Peak airway pressure was lower, but mean airway pressure was higher with APRV. There were no statistically significant differences in gas exchange or hemodynamic variables.
Putensen et al,33 in a study designed on the basis of prior publications,15 randomized 30 patients with multiple trauma to either APRV with spontaneous breathing (n = 15) or pressure-control ventilation (n = 15) for 72 hours. Weaning was performed with APRV in both groups. APRV was associated with increases in lung compliance and oxygenation and reduction of shunting. Interestingly, the use of APRV was associated with shorter duration of ventilatory support (15 vs 21 days), shorter length of intensive care unit stay (23 vs 30 days), and shorter duration of sedation and use of vasopressors.
An important confounder in this trial was that all patients on pressure-control ventilation were initially paralyzed, favoring the APRV group.
Varpula and colleagues34 performed a prospective randomized intervention study to determine whether the response of oxygenation to the prone position differed between APRV vs pressure-controlled synchronized intermittent mandatory ventilation with pressure support. Forty-five patients with ALI were randomized within 72 hours of initiation of mechanical ventilation to receive one of these two modes; 33 ultimately received the assigned treatment. All patients were positioned on their stomachs for 6 hours once or twice a day. The response in terms of oxygenation to the first pronation was similar in both groups, whereas there was a significant improvement after the second pronation in the APRV group. The authors concluded that prone positioning and allowance of spontaneous breathing during APRV had advantageous effects on gas exchange.
In 2004, the same investigators35 randomized 58 patients with ALI after stabilization to either APRV or pressure-controlled synchronized intermittent mandatory ventilation. There were no significant differences in the clinically important outcomes such as ventilator-free days, sedation days, need of hemodialysis, or intensive care unit-free days.
Dart et al,36 in a retrospective study of 46 trauma patients who were ventilated with APRV for 72 hours, found an improvement in the Pao2/Fio2 ratio and a decrement in peak airway pressure after APRV was started.
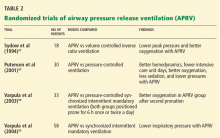
Table 2 summarizes the randomized clinical trials of APRV.33–35,37
CONCERNS ABOUT APRV
Overstretching. One of the major concerns when applying APRV is overstretching the lung parenchyma.26,38 It is important to recognize that, when choosing a P high setting, this variable is not the only determinant of the tidal volume. Spontaneous breathing causes the pleural pressure to become less positive. As a result, there is an increase in the transpulmonary pressure (pressure in alveoli minus pressure in the pleura). This augmentation of transpulmonary pressure will result in a higher tidal volume and the risk of overdistention and volume-induced lung injury.
Atelectrauma. As mentioned earlier, damage may occur when airways open and close with each tidal cycle. This is particularly worrisome when the end-expiratory pressure is below the lower inflection point, as some diseased alveolar units may collapse. In APRV, the airway pressure is released to zero. Even though the intentional auto-PEEP might maintain a certain end-expiratory pressure, this parameter is truly uncontrolled.39
If the patient cannot breath spontaneously. Another consideration is that many of the benefits of APRV are based on the spontaneous breathing component. Unfortunately, patients who need heavy sedation or neuromuscular paralysis with lack of spontaneous breathing efforts may lose the physiologic advantages of this mode.
Despite these limitations, APRV presents many attractive benefits as an alternative mode of mechanical ventilation in patients who do not respond to conventional modes.
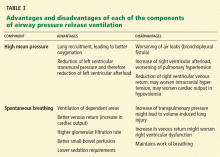
Table 3 summarizes the advantages and disadvantages of each component of APRV.
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992; 18:319–321.
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–1786.
- Papadakos PJ, Lachmann B. The open lung concept of mechanical ventilation: the role of recruitment and stabilization. Crit Care Clin 2007; 23:241–250,
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Dreyfuss D, Saumon G, Hubmayr RD, editors. Ventilator-induced Lung Injury. New York: Taylor & Francis, 2006.
- Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006; 34:1311–1318.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Parsons PE, Eisner MD, Thompson BT, et al; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33:1–6.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354.
- Hemmila MR, Napolitano LM. Severe respiratory failure: advanced treatment options. Crit Care Med 2006; 34( suppl 9):S278–S290.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Martin LD, Wetzel RC. Optimal release time during airway pressure release ventilation in neonatal sheep. Crit Care Med 1994; 22:486–493.
- Frawley PM, Habashi NM. Airway pressure release ventilation: theory and practice. AACN Clin Issues 2001; 12:234–246.
- Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 159:1241–1248.
- Putensen C, Wrigge H. Clinical review: biphasic positive airway pressure and airway pressure release ventilation. Crit Care 2004; 8:492–497.
- Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33( suppl 3):S228–S240.
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41:242–255.
- Reber A, Nylund U, Hedenstierna G. Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia 1998; 53:1054–1061.
- Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158:1644–1655.
- Gattinoni L, Presenti A, Torresin A, et al. Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1986; 1:25–30.
- Hedenstierna G, Lichtwarck-Aschoff M. Interfacing spontaneous breathing and mechanical ventilation. New insights. Minerva Anestesiol 2006; 72:183–198.
- Neumann P, Wrigge H, Zinserling J, et al. Spontaneous breathing affects the spatial ventilation and perfusion distribution during mechanical ventilatory support. Crit Care Med 2005; 33:1090–1095.
- Räsänen J, Downs JB, Stock MC. Cardiovascular effects of conventional positive pressure ventilation and airway pressure release ventilation. Chest 1988; 93:911–915.
- Falkenhain SK, Reilley TE, Gregory JS. Improvement in cardiac output during airway pressure release ventilation. Crit Care Med 1992; 20:1358–1360.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med 2002; 28:1426–1433.
- Hering R, Viehöfer A, Zinserling J, et al. Effects of spontaneous breathing during airway pressure release ventilation on intestinal blood flow in experimental lung injury. Anesthesiology 2003; 99:1137–1144.
- Martin LD, Wetzel RC, Bilenki AL. Airway pressure release ventilation in a neonatal lamb model of acute lung injury. Crit Care Med 1991; 19:373–378.
- Garner W, Downs JB, Stock MC, Räsänen J. Airway pressure release ventilation (APRV). A human trial. Chest 1988; 94:779–781.
- Räsänen J, Cane RD, Downs JB, et al. Airway pressure release ventilation during acute lung injury: a prospective multicenter trial. Crit Care Med 1991; 19:1234–1241.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Varpula T, Jousela I, Niemi R, Takkunen O, Pettilä V. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand 2003; 47:516–524.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Dart BW, Maxwell RA, Richart CM, et al. Preliminary experience with airway pressure release ventilation in a trauma/surgical intensive care unit. J Trauma 2005; 59:71–76.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Dries DJ, Marini JJ. Airway pressure release ventilation. J Burn Care Res 2009; 30:929–936.
In the early stages of acute respiratory distress syndrome (ARDS), multiple areas of the lung collapse, most often in the dependent regions. A factor involved in this process is the loss of functional surfactant, creating a condition in which alveolar units are unstable and prone to collapse due to unopposed surface tension. This situation, similar to that in premature infants, results in a reduced volume of aerated lung, intrapulmonary shunting, and, therefore, poor oxygenation.
The treatment of this alveolar collapse is lung reinflation (or “recruitment,” a term first used by Lachmann).1 Gattinoni et al2 showed that the percentage of recruitable lung could range from a negligible fraction to 50% or more.
There are various means of reopening injured lungs and keeping them open. The choice of recruitment maneuver is based on the individual patient and the ventilatory mode.3
In this article, we review airway pressure release ventilation (APRV), a mode of mechanical ventilation that may be useful in situations in which, due to ARDS, the lungs need to be recruited and held open. APRV was developed as a lung-protective mode, allowing recruitment while minimizing ventilator-induced lung injury.
BASIC PRINCIPLES OF PROTECTIVE VENTILATION
This curve has two inflection points between which its slope is steep, indicating greater compliance or elasticity. Below the lower inflection point, the alveoli may collapse; above the upper inflection point, the lung loses its elastic properties and the alveoli are overdistended. To protect the lungs, the challenge in mechanical ventilation is to keep the lungs between these two points throughout the respiratory cycle.
Avoiding lung collapse by using PEEP
During mechanical ventilation, the pressure in the lungs is lowest, and thus the alveoli are most prone to collapse, at the end of expiration.
We want to prevent the alveoli from collapsing with each expiration and reopening with each inspiration, as this cycle of opening and closing damages them (causing atelectrauma, ie, cyclical atelectasis).4 Preventing it prevents the release of inflammatory mediators and the perpetuation of lung injury (biotrauma).5
The solution is to apply positive end-expiratory pressure (PEEP), taking into account the value of the lower inflection point when setting the PEEP level.
Villar et al6 compared outcomes in an intervention group that received a PEEP level 2 cm H2O above the lower inflection point plus low tidal volumes, and in a control group that received higher tidal volumes and low PEEP (5 cm H2O). The study was stopped early, after significantly more patients had died in the control group than in the intervention group (53% vs 32%, P = .04).
Avoiding overdistention by keeping the tidal volume low
Tidal volumes that exceed the upper inflection point overstretch the lung and induce volutrauma, which can manifest as pneumothorax or pneumomediastinum, or both—the lungs rupture like a balloon. Also, overdistention produces liberation of inflammatory mediators in the blood (biotrauma). High tidal volumes should therefore be avoided or limited as much as possible.
The ARDS Network,7 in a multicenter, randomized, controlled trial, showed that fewer patients die if they receive mechanical ventilation with low tidal volumes rather than higher, “conventional” tidal volumes. Patients were randomized to receive either a tidal volume of 6 mL/kg and a plateau pressure lower than 30 cm H2O or a tidal volume of 12 mL/kg and a plateau pressure lower than 50 cm H2O. They were followed for 180 days or until discharged home, breathing without assistance. A total of 861 patients were enrolled. The mortality rate was significantly lower in the low tidal volume group than in the group with conventional tidal volumes, 31% vs 40%.
Lower tidal volumes were also associated with faster attenuation of the inflammatory response.8
Amato et al9 randomized 58 patients to receive mechanical ventilation with tidal volumes of either 6 mL/kg or 12 mL/kg. The PEEP level was maintained above the lower inflection point. At 28 days, 62% of the patients in the intervention group were still alive, compared with only 29% in the control group. However, many concerns were expressed over the high mortality rate in the control group.
Based on these studies, the use of low tidal volumes with appropriate levels of PEEP to ensure lung recruitment is the current standard of care in mechanical ventilation of patients with ARDS.10
APRV: A PRESSURE-CONTROLLED MODE THAT ALLOWS SPONTANEOUS BREATHS
A baseline high pressure (P high) is set first. Mandatory breaths are achieved by releasing the high baseline pressure in the circuit very briefly, usually to 0 cm H2O (P low), which allows the lungs to partially deflate, and then quickly resuming the high pressure before the unstable alveoli can collapse.
In theory, the optimal release time (the very short time in low pressure, or T low) in APRV should be determined by the time constant of the expiratory flow. The time constant (t) is the time it takes to empty 63% of the lung volume. It is calculated as:
t = C × R
where C is the combined compliance of the lung and chest wall, and R is the combined resistance of the endotracheal tube and the natural airways. In diseases that lead to lower lung compliance (such as ARDS), the time constant is shorter. A practical equilibrium time—or the time it takes for the lung volume in expiration to reach steady state (no expiratory flow)—is about 4 time constants.14
Since the release time in APRV is much shorter than the equilibrium time, a residual volume of air remains in the lung, creating intentional auto-PEEP. Ideally, this intentional auto-PEEP should be high enough to avoid derecruitment (optimally above the lower inflection point). In APRV the auto-PEEP is controlled by the settings, and this intentional restriction of the expiratory flow is critical to avoid derecruitment of unstable alveolar units.
The amount of time spent at the higher pressure (T high) is generally 80% to 95% of the cycle (ie, the lungs are “inflated” 80% to 95% of the time), and the amount of time at the lower pressure (T low) is 0.6 to 0.8 seconds.
Thus, APRV settings provide a relatively high mean airway pressure, which prevents collapse of unstable alveoli and over time recruits additional alveolar units in the injured lung. The major difference between this mode and more conventional modes is that in APRV the mean inspiratory pressure is maximized and end-expiratory pressure is due to intentional auto-PEEP. In addition, spontaneous breathing is allowed throughout the entire cycle (Figure 2).13
Although APRV does not approximate the physiology of spontaneous breathing with healthy lungs, it is nonetheless relatively comfortable and well tolerated. Its theoretical advantage in patients with lung injury is its ability to maximize alveoli recruitment by maintaining a higher mean inspiratory pressure, while the peak alveolar pressure remains lower than with conventional ventilation (Figure 1).
Other modes that are similar to APRV
Other modes of mechanical ventilation very similar to APRV are biphasic positive airway pressure (BiPAP) and bilevel ventilation.
BiPAP differs from APRV only in the timing of the upper and lower pressure levels. In BiPAP, T high is usually shorter than T low. Therefore, in order to avoid derecruitment, P low has to be set above zero with both a high and a low PEEP level.13
No studies have demonstrated one mode to be more beneficial than the other, although BiPAP might be more predictable, as both pressures are known.
Bilevel ventilation works like APRV but incorporates pressure support to spontaneous breathing. The use of pressure support may affect the positive physiologic effects (see section below) of unsupported spontaneous breathing. Nevertheless, this strategy might be useful to address severe hypercapnia in the context of APRV.
INITIAL VENTILATOR SETTINGS IN APRV
P high. In selecting an initial P high, we measure the plateau pressure in a conventional mode using an accepted protective strategy, such as volume-control mode. If the plateau pressure is lower than 30 cm H2O, we use this pressure as our initial P high. If the plateau pressure is higher than 30 cm H2O, we select 30 cm H2O as an initial P high to minimize peak alveolar pressure and reduce the risk of lung overdistention.
P low is set at 0 cm H2O.
T high is set at 4 seconds and is then adjusted if necessary.
T low is probably the most difficult variable to set because it needs to be short enough to avoid derecruitment but still long enough to allow alveolar ventilation. We usually start with a T low of 0.6 to 0.8 seconds.
ADJUSTING THE VENTILATOR SETTINGS
For hypoxemia. Physician-controlled variables that affect oxygenation in APRV are:
- Mean airway pressure (dependent primarily on P high and T high)
- Fraction of inspired oxygen (Fio2).
Inadequate oxygenation usually requires increasing one or both of these settings.
Physician-controlled variables that affect alveolar ventilation in the APRV mode are:
- Pressure gradient (P high minus P low)
- Airway pressure release time (T low)
- Airway pressure release frequency.14 Frequency is related to total cycle time of mandatory breaths by the following equation3:
frequency = 60/cycle time = 60/(T high + T low).
Note that if T low remains constant, adjusting T high will adjust frequency (the more time the lung remains inflated, the lower the respiratory frequency). Conversely, some ventilators allow adjustment of frequency, making T high the dependent variable. The goal of this mode is to recruit alveoli and improve oxygenation, so we usually do not modify the pressure gradient to improve ventilation.
For hypercapnia. A frequent and expected consequence of lung-protective ventilation strategies is hypercapnia, termed “permissive” hypercapnia because it is allowed to some extent. In APRV, some degree of CO2 retention is not unusual. When the measured Paco2 becomes extreme, we usually increase the frequency of releases by shortening T high, recognizing that this adjustment may affect recruitment by lowering the mean airway pressure.
Spontaneous breaths. A positive aspect of APRV that contributes to its tolerability for patients is that it allows for spontaneous respiration. In some studies of patients with ARDS ventilated with APRV, spontaneous breathing accounted for 10% to 30% of the total minute ventilation and was responsible for an improvement in ventilation-perfusion matching and oxygenation.15,16 We titrate our patients’ sedation to a goal of spontaneous breathing of at least 10% of total minute ventilation.
WEANING FROM APRV
Weaning from APRV is done carefully to avoid derecruitment. Some authors recommend lowering P high by 2 to 3 cm H2O at a time and lengthening T high by increments of 0.5 to 2.0 seconds.13,17
Once P high is about 16 cm H2O, T high is at 12 to 15 seconds, and spontaneous respiration accounts for most or all of the minute volume, the mode can be changed to continuous positive airway pressure (CPAP) and titrated downwards. Usually, when CPAP is at 5 to 10 cm H2O, the patient is extubated, provided that mental status or concerns about airway protection or secretions are not contraindications.
PHYSIOLOGIC EFFECTS OF APRV WITH SPONTANEOUS BREATHING
Effects on the respiratory system
During spontaneous breathing, the greatest displacement of the diaphragm is in dependent regions. These regions are the best ventilated.18 Compared with spontaneously breathing patients, mechanically ventilated patients have a smaller inspiratory displacement of the dependent part of the lung.19
A study using computed tomography demonstrated that the reduction of lung volume observed in patients with acute lung injury (ALI) predominantly affects the lower lobes (dependent areas).20 Causative mechanisms could be an increase in lung weight related to ALI and a passive collapse of the lower lobes associated with an upward shift of the diaphragm.
In a preliminary study, the topographic distribution of lung collapse was different in spontaneously breathing ARDS patients than in patients who were paralyzed. In particular, lung densities were not concentrated in the dependent regions in the former group.21
Oxygenation is better with APRV with spontaneous breathing than with mechanical ventilation alone. This effect is at least partly attributable to recruitment of collapsed lung tissue and increased aeration of the dependent areas of the lung.22
Putensen et al15 compared ventilation-perfusion distribution in 24 patients with ARDS who were randomized to APRV with spontaneous breathing (more than 10% of the total minute ventilation), APRV without spontaneous breathing, or pressure-support ventilation. Spontaneous breathing during APRV improved ventilation-perfusion matching and increased systemic blood flow.
Neumann et al23 recently compared the effect of APRV with spontaneous breathing vs APRV without spontaneous breathing in terms of ventilation perfusion in an animal model of lung injury. APRV with spontaneous breathing increased ventilation in juxta-diaphragmatic regions, predominantly in dependent areas. Spontaneous breathing had a significant effect on the spatial distribution of ventilation and pulmonary perfusion.
Based on these studies, we generally use APRV with no pressure support. This strategy permits recruitment and expansion of dependent lung areas.
Effects on the cardiovascular system and hemodynamics
Räsänen et al,24 in an animal model, compared cardiovascular performance during APRV, spontaneous breathing, and continuous positive pressure ventilation. No significant differences in cardiovascular function were detected between APRV and spontaneous breathing. In contrast, continuous positive pressure ventilation decreased blood pressure, stroke volume, cardiac output, and oxygen delivery.
Falkenhain et al,25 in a subsequent case report, found that a change in mode from intermittent mandatory ventilation with PEEP to APRV resulted in improvement in the cardiac output of a patient requiring mechanical ventilation.
The lack of deleterious effect of APRV on cardiovascular function is probably a result of its spontaneous breathing component. The reduction in mean intrathoracic pressure during spontaneous breathing (compared to paralysis) improves venous return and biventricular filling, boosting cardiac output and oxygen delivery.26
Hering et al27 compared APRV with spontaneous breathing (at least 30% of the total minute ventilation) vs APRV with no spontaneous breathing in 12 patients with ALI. This study showed higher renal blood flow, glomerular filtration, and osmolar clearance in the APRV-with-spontaneous-breathing group.
The same investigators evaluated the effects of spontaneous breathing with APRV on intestinal blood flow in an animal model of lung injury.28 Spontaneous breathing with APRV improved arterial oxygenation, the systemic hemodynamic profile, and regional perfusion to the stomach and small bowel compared with full ventilatory support.
ANIMAL STUDIES OF APRV
Stock et al,11 in their original description of APRV in 1987, reported experimental results in dogs. In that study, 10 dogs with and without ARDS were randomized to APRV with a custom-built device vs volume-control mode with a Harvard pump ventilator plus PEEP. APRV delivered adequate alveolar ventilation, had lower peak airway pressures, and promoted better arterial oxygenation (at the same tidal volume and mean airway pressure) compared with volume control.
Martin et al (1991)29 studied seven neonatal lambs with ALI with four ventilatory modes: pressure-support ventilation, APRV, volume control, and spontaneous breathing. APRV maintained oxygenation while augmenting alveolar ventilation compared with pressure-support ventilation. APRV also provided ventilation at a lower peak pressure in contrast to volume control. The authors concluded that APRV was an effective mode to maintain oxygenation and assist alveolar ventilation with minimal cardiovascular impact in their animal model of ALI.
HUMAN STUDIES OF APRV
Garner et al (1988)30 studied 14 patients after operative coronary revascularization, giving them volume control mode (12 mL/kg) and then, when they were hemodynamically stable, APRV. While APRV and volume control supported ventilation and arterial oxygenation equally in all cases, peak airway pressure was greater with volume control.
Räsänen et al (1991)31 designed a prospective, multicenter, crossover trial in which 50 patients with ALI were ventilated with conventional ventilation and subsequently with APRV. Patients in both groups were adequately ventilated and oxygenated. However, as described in the aforementioned study,24 the peak airway pressure was lower in the APRV group.
Davis et al (1993)32 studied 15 patients with ARDS requiring ventilatory support who received intermittent mandatory ventilation plus PEEP and then were placed on APRV. Peak airway pressure was lower, but mean airway pressure was higher with APRV. There were no statistically significant differences in gas exchange or hemodynamic variables.
Putensen et al,33 in a study designed on the basis of prior publications,15 randomized 30 patients with multiple trauma to either APRV with spontaneous breathing (n = 15) or pressure-control ventilation (n = 15) for 72 hours. Weaning was performed with APRV in both groups. APRV was associated with increases in lung compliance and oxygenation and reduction of shunting. Interestingly, the use of APRV was associated with shorter duration of ventilatory support (15 vs 21 days), shorter length of intensive care unit stay (23 vs 30 days), and shorter duration of sedation and use of vasopressors.
An important confounder in this trial was that all patients on pressure-control ventilation were initially paralyzed, favoring the APRV group.
Varpula and colleagues34 performed a prospective randomized intervention study to determine whether the response of oxygenation to the prone position differed between APRV vs pressure-controlled synchronized intermittent mandatory ventilation with pressure support. Forty-five patients with ALI were randomized within 72 hours of initiation of mechanical ventilation to receive one of these two modes; 33 ultimately received the assigned treatment. All patients were positioned on their stomachs for 6 hours once or twice a day. The response in terms of oxygenation to the first pronation was similar in both groups, whereas there was a significant improvement after the second pronation in the APRV group. The authors concluded that prone positioning and allowance of spontaneous breathing during APRV had advantageous effects on gas exchange.
In 2004, the same investigators35 randomized 58 patients with ALI after stabilization to either APRV or pressure-controlled synchronized intermittent mandatory ventilation. There were no significant differences in the clinically important outcomes such as ventilator-free days, sedation days, need of hemodialysis, or intensive care unit-free days.
Dart et al,36 in a retrospective study of 46 trauma patients who were ventilated with APRV for 72 hours, found an improvement in the Pao2/Fio2 ratio and a decrement in peak airway pressure after APRV was started.
Table 2 summarizes the randomized clinical trials of APRV.33–35,37
CONCERNS ABOUT APRV
Overstretching. One of the major concerns when applying APRV is overstretching the lung parenchyma.26,38 It is important to recognize that, when choosing a P high setting, this variable is not the only determinant of the tidal volume. Spontaneous breathing causes the pleural pressure to become less positive. As a result, there is an increase in the transpulmonary pressure (pressure in alveoli minus pressure in the pleura). This augmentation of transpulmonary pressure will result in a higher tidal volume and the risk of overdistention and volume-induced lung injury.
Atelectrauma. As mentioned earlier, damage may occur when airways open and close with each tidal cycle. This is particularly worrisome when the end-expiratory pressure is below the lower inflection point, as some diseased alveolar units may collapse. In APRV, the airway pressure is released to zero. Even though the intentional auto-PEEP might maintain a certain end-expiratory pressure, this parameter is truly uncontrolled.39
If the patient cannot breath spontaneously. Another consideration is that many of the benefits of APRV are based on the spontaneous breathing component. Unfortunately, patients who need heavy sedation or neuromuscular paralysis with lack of spontaneous breathing efforts may lose the physiologic advantages of this mode.
Despite these limitations, APRV presents many attractive benefits as an alternative mode of mechanical ventilation in patients who do not respond to conventional modes.
Table 3 summarizes the advantages and disadvantages of each component of APRV.
In the early stages of acute respiratory distress syndrome (ARDS), multiple areas of the lung collapse, most often in the dependent regions. A factor involved in this process is the loss of functional surfactant, creating a condition in which alveolar units are unstable and prone to collapse due to unopposed surface tension. This situation, similar to that in premature infants, results in a reduced volume of aerated lung, intrapulmonary shunting, and, therefore, poor oxygenation.
The treatment of this alveolar collapse is lung reinflation (or “recruitment,” a term first used by Lachmann).1 Gattinoni et al2 showed that the percentage of recruitable lung could range from a negligible fraction to 50% or more.
There are various means of reopening injured lungs and keeping them open. The choice of recruitment maneuver is based on the individual patient and the ventilatory mode.3
In this article, we review airway pressure release ventilation (APRV), a mode of mechanical ventilation that may be useful in situations in which, due to ARDS, the lungs need to be recruited and held open. APRV was developed as a lung-protective mode, allowing recruitment while minimizing ventilator-induced lung injury.
BASIC PRINCIPLES OF PROTECTIVE VENTILATION
This curve has two inflection points between which its slope is steep, indicating greater compliance or elasticity. Below the lower inflection point, the alveoli may collapse; above the upper inflection point, the lung loses its elastic properties and the alveoli are overdistended. To protect the lungs, the challenge in mechanical ventilation is to keep the lungs between these two points throughout the respiratory cycle.
Avoiding lung collapse by using PEEP
During mechanical ventilation, the pressure in the lungs is lowest, and thus the alveoli are most prone to collapse, at the end of expiration.
We want to prevent the alveoli from collapsing with each expiration and reopening with each inspiration, as this cycle of opening and closing damages them (causing atelectrauma, ie, cyclical atelectasis).4 Preventing it prevents the release of inflammatory mediators and the perpetuation of lung injury (biotrauma).5
The solution is to apply positive end-expiratory pressure (PEEP), taking into account the value of the lower inflection point when setting the PEEP level.
Villar et al6 compared outcomes in an intervention group that received a PEEP level 2 cm H2O above the lower inflection point plus low tidal volumes, and in a control group that received higher tidal volumes and low PEEP (5 cm H2O). The study was stopped early, after significantly more patients had died in the control group than in the intervention group (53% vs 32%, P = .04).
Avoiding overdistention by keeping the tidal volume low
Tidal volumes that exceed the upper inflection point overstretch the lung and induce volutrauma, which can manifest as pneumothorax or pneumomediastinum, or both—the lungs rupture like a balloon. Also, overdistention produces liberation of inflammatory mediators in the blood (biotrauma). High tidal volumes should therefore be avoided or limited as much as possible.
The ARDS Network,7 in a multicenter, randomized, controlled trial, showed that fewer patients die if they receive mechanical ventilation with low tidal volumes rather than higher, “conventional” tidal volumes. Patients were randomized to receive either a tidal volume of 6 mL/kg and a plateau pressure lower than 30 cm H2O or a tidal volume of 12 mL/kg and a plateau pressure lower than 50 cm H2O. They were followed for 180 days or until discharged home, breathing without assistance. A total of 861 patients were enrolled. The mortality rate was significantly lower in the low tidal volume group than in the group with conventional tidal volumes, 31% vs 40%.
Lower tidal volumes were also associated with faster attenuation of the inflammatory response.8
Amato et al9 randomized 58 patients to receive mechanical ventilation with tidal volumes of either 6 mL/kg or 12 mL/kg. The PEEP level was maintained above the lower inflection point. At 28 days, 62% of the patients in the intervention group were still alive, compared with only 29% in the control group. However, many concerns were expressed over the high mortality rate in the control group.
Based on these studies, the use of low tidal volumes with appropriate levels of PEEP to ensure lung recruitment is the current standard of care in mechanical ventilation of patients with ARDS.10
APRV: A PRESSURE-CONTROLLED MODE THAT ALLOWS SPONTANEOUS BREATHS
A baseline high pressure (P high) is set first. Mandatory breaths are achieved by releasing the high baseline pressure in the circuit very briefly, usually to 0 cm H2O (P low), which allows the lungs to partially deflate, and then quickly resuming the high pressure before the unstable alveoli can collapse.
In theory, the optimal release time (the very short time in low pressure, or T low) in APRV should be determined by the time constant of the expiratory flow. The time constant (t) is the time it takes to empty 63% of the lung volume. It is calculated as:
t = C × R
where C is the combined compliance of the lung and chest wall, and R is the combined resistance of the endotracheal tube and the natural airways. In diseases that lead to lower lung compliance (such as ARDS), the time constant is shorter. A practical equilibrium time—or the time it takes for the lung volume in expiration to reach steady state (no expiratory flow)—is about 4 time constants.14
Since the release time in APRV is much shorter than the equilibrium time, a residual volume of air remains in the lung, creating intentional auto-PEEP. Ideally, this intentional auto-PEEP should be high enough to avoid derecruitment (optimally above the lower inflection point). In APRV the auto-PEEP is controlled by the settings, and this intentional restriction of the expiratory flow is critical to avoid derecruitment of unstable alveolar units.
The amount of time spent at the higher pressure (T high) is generally 80% to 95% of the cycle (ie, the lungs are “inflated” 80% to 95% of the time), and the amount of time at the lower pressure (T low) is 0.6 to 0.8 seconds.
Thus, APRV settings provide a relatively high mean airway pressure, which prevents collapse of unstable alveoli and over time recruits additional alveolar units in the injured lung. The major difference between this mode and more conventional modes is that in APRV the mean inspiratory pressure is maximized and end-expiratory pressure is due to intentional auto-PEEP. In addition, spontaneous breathing is allowed throughout the entire cycle (Figure 2).13
Although APRV does not approximate the physiology of spontaneous breathing with healthy lungs, it is nonetheless relatively comfortable and well tolerated. Its theoretical advantage in patients with lung injury is its ability to maximize alveoli recruitment by maintaining a higher mean inspiratory pressure, while the peak alveolar pressure remains lower than with conventional ventilation (Figure 1).
Other modes that are similar to APRV
Other modes of mechanical ventilation very similar to APRV are biphasic positive airway pressure (BiPAP) and bilevel ventilation.
BiPAP differs from APRV only in the timing of the upper and lower pressure levels. In BiPAP, T high is usually shorter than T low. Therefore, in order to avoid derecruitment, P low has to be set above zero with both a high and a low PEEP level.13
No studies have demonstrated one mode to be more beneficial than the other, although BiPAP might be more predictable, as both pressures are known.
Bilevel ventilation works like APRV but incorporates pressure support to spontaneous breathing. The use of pressure support may affect the positive physiologic effects (see section below) of unsupported spontaneous breathing. Nevertheless, this strategy might be useful to address severe hypercapnia in the context of APRV.
INITIAL VENTILATOR SETTINGS IN APRV
P high. In selecting an initial P high, we measure the plateau pressure in a conventional mode using an accepted protective strategy, such as volume-control mode. If the plateau pressure is lower than 30 cm H2O, we use this pressure as our initial P high. If the plateau pressure is higher than 30 cm H2O, we select 30 cm H2O as an initial P high to minimize peak alveolar pressure and reduce the risk of lung overdistention.
P low is set at 0 cm H2O.
T high is set at 4 seconds and is then adjusted if necessary.
T low is probably the most difficult variable to set because it needs to be short enough to avoid derecruitment but still long enough to allow alveolar ventilation. We usually start with a T low of 0.6 to 0.8 seconds.
ADJUSTING THE VENTILATOR SETTINGS
For hypoxemia. Physician-controlled variables that affect oxygenation in APRV are:
- Mean airway pressure (dependent primarily on P high and T high)
- Fraction of inspired oxygen (Fio2).
Inadequate oxygenation usually requires increasing one or both of these settings.
Physician-controlled variables that affect alveolar ventilation in the APRV mode are:
- Pressure gradient (P high minus P low)
- Airway pressure release time (T low)
- Airway pressure release frequency.14 Frequency is related to total cycle time of mandatory breaths by the following equation3:
frequency = 60/cycle time = 60/(T high + T low).
Note that if T low remains constant, adjusting T high will adjust frequency (the more time the lung remains inflated, the lower the respiratory frequency). Conversely, some ventilators allow adjustment of frequency, making T high the dependent variable. The goal of this mode is to recruit alveoli and improve oxygenation, so we usually do not modify the pressure gradient to improve ventilation.
For hypercapnia. A frequent and expected consequence of lung-protective ventilation strategies is hypercapnia, termed “permissive” hypercapnia because it is allowed to some extent. In APRV, some degree of CO2 retention is not unusual. When the measured Paco2 becomes extreme, we usually increase the frequency of releases by shortening T high, recognizing that this adjustment may affect recruitment by lowering the mean airway pressure.
Spontaneous breaths. A positive aspect of APRV that contributes to its tolerability for patients is that it allows for spontaneous respiration. In some studies of patients with ARDS ventilated with APRV, spontaneous breathing accounted for 10% to 30% of the total minute ventilation and was responsible for an improvement in ventilation-perfusion matching and oxygenation.15,16 We titrate our patients’ sedation to a goal of spontaneous breathing of at least 10% of total minute ventilation.
WEANING FROM APRV
Weaning from APRV is done carefully to avoid derecruitment. Some authors recommend lowering P high by 2 to 3 cm H2O at a time and lengthening T high by increments of 0.5 to 2.0 seconds.13,17
Once P high is about 16 cm H2O, T high is at 12 to 15 seconds, and spontaneous respiration accounts for most or all of the minute volume, the mode can be changed to continuous positive airway pressure (CPAP) and titrated downwards. Usually, when CPAP is at 5 to 10 cm H2O, the patient is extubated, provided that mental status or concerns about airway protection or secretions are not contraindications.
PHYSIOLOGIC EFFECTS OF APRV WITH SPONTANEOUS BREATHING
Effects on the respiratory system
During spontaneous breathing, the greatest displacement of the diaphragm is in dependent regions. These regions are the best ventilated.18 Compared with spontaneously breathing patients, mechanically ventilated patients have a smaller inspiratory displacement of the dependent part of the lung.19
A study using computed tomography demonstrated that the reduction of lung volume observed in patients with acute lung injury (ALI) predominantly affects the lower lobes (dependent areas).20 Causative mechanisms could be an increase in lung weight related to ALI and a passive collapse of the lower lobes associated with an upward shift of the diaphragm.
In a preliminary study, the topographic distribution of lung collapse was different in spontaneously breathing ARDS patients than in patients who were paralyzed. In particular, lung densities were not concentrated in the dependent regions in the former group.21
Oxygenation is better with APRV with spontaneous breathing than with mechanical ventilation alone. This effect is at least partly attributable to recruitment of collapsed lung tissue and increased aeration of the dependent areas of the lung.22
Putensen et al15 compared ventilation-perfusion distribution in 24 patients with ARDS who were randomized to APRV with spontaneous breathing (more than 10% of the total minute ventilation), APRV without spontaneous breathing, or pressure-support ventilation. Spontaneous breathing during APRV improved ventilation-perfusion matching and increased systemic blood flow.
Neumann et al23 recently compared the effect of APRV with spontaneous breathing vs APRV without spontaneous breathing in terms of ventilation perfusion in an animal model of lung injury. APRV with spontaneous breathing increased ventilation in juxta-diaphragmatic regions, predominantly in dependent areas. Spontaneous breathing had a significant effect on the spatial distribution of ventilation and pulmonary perfusion.
Based on these studies, we generally use APRV with no pressure support. This strategy permits recruitment and expansion of dependent lung areas.
Effects on the cardiovascular system and hemodynamics
Räsänen et al,24 in an animal model, compared cardiovascular performance during APRV, spontaneous breathing, and continuous positive pressure ventilation. No significant differences in cardiovascular function were detected between APRV and spontaneous breathing. In contrast, continuous positive pressure ventilation decreased blood pressure, stroke volume, cardiac output, and oxygen delivery.
Falkenhain et al,25 in a subsequent case report, found that a change in mode from intermittent mandatory ventilation with PEEP to APRV resulted in improvement in the cardiac output of a patient requiring mechanical ventilation.
The lack of deleterious effect of APRV on cardiovascular function is probably a result of its spontaneous breathing component. The reduction in mean intrathoracic pressure during spontaneous breathing (compared to paralysis) improves venous return and biventricular filling, boosting cardiac output and oxygen delivery.26
Hering et al27 compared APRV with spontaneous breathing (at least 30% of the total minute ventilation) vs APRV with no spontaneous breathing in 12 patients with ALI. This study showed higher renal blood flow, glomerular filtration, and osmolar clearance in the APRV-with-spontaneous-breathing group.
The same investigators evaluated the effects of spontaneous breathing with APRV on intestinal blood flow in an animal model of lung injury.28 Spontaneous breathing with APRV improved arterial oxygenation, the systemic hemodynamic profile, and regional perfusion to the stomach and small bowel compared with full ventilatory support.
ANIMAL STUDIES OF APRV
Stock et al,11 in their original description of APRV in 1987, reported experimental results in dogs. In that study, 10 dogs with and without ARDS were randomized to APRV with a custom-built device vs volume-control mode with a Harvard pump ventilator plus PEEP. APRV delivered adequate alveolar ventilation, had lower peak airway pressures, and promoted better arterial oxygenation (at the same tidal volume and mean airway pressure) compared with volume control.
Martin et al (1991)29 studied seven neonatal lambs with ALI with four ventilatory modes: pressure-support ventilation, APRV, volume control, and spontaneous breathing. APRV maintained oxygenation while augmenting alveolar ventilation compared with pressure-support ventilation. APRV also provided ventilation at a lower peak pressure in contrast to volume control. The authors concluded that APRV was an effective mode to maintain oxygenation and assist alveolar ventilation with minimal cardiovascular impact in their animal model of ALI.
HUMAN STUDIES OF APRV
Garner et al (1988)30 studied 14 patients after operative coronary revascularization, giving them volume control mode (12 mL/kg) and then, when they were hemodynamically stable, APRV. While APRV and volume control supported ventilation and arterial oxygenation equally in all cases, peak airway pressure was greater with volume control.
Räsänen et al (1991)31 designed a prospective, multicenter, crossover trial in which 50 patients with ALI were ventilated with conventional ventilation and subsequently with APRV. Patients in both groups were adequately ventilated and oxygenated. However, as described in the aforementioned study,24 the peak airway pressure was lower in the APRV group.
Davis et al (1993)32 studied 15 patients with ARDS requiring ventilatory support who received intermittent mandatory ventilation plus PEEP and then were placed on APRV. Peak airway pressure was lower, but mean airway pressure was higher with APRV. There were no statistically significant differences in gas exchange or hemodynamic variables.
Putensen et al,33 in a study designed on the basis of prior publications,15 randomized 30 patients with multiple trauma to either APRV with spontaneous breathing (n = 15) or pressure-control ventilation (n = 15) for 72 hours. Weaning was performed with APRV in both groups. APRV was associated with increases in lung compliance and oxygenation and reduction of shunting. Interestingly, the use of APRV was associated with shorter duration of ventilatory support (15 vs 21 days), shorter length of intensive care unit stay (23 vs 30 days), and shorter duration of sedation and use of vasopressors.
An important confounder in this trial was that all patients on pressure-control ventilation were initially paralyzed, favoring the APRV group.
Varpula and colleagues34 performed a prospective randomized intervention study to determine whether the response of oxygenation to the prone position differed between APRV vs pressure-controlled synchronized intermittent mandatory ventilation with pressure support. Forty-five patients with ALI were randomized within 72 hours of initiation of mechanical ventilation to receive one of these two modes; 33 ultimately received the assigned treatment. All patients were positioned on their stomachs for 6 hours once or twice a day. The response in terms of oxygenation to the first pronation was similar in both groups, whereas there was a significant improvement after the second pronation in the APRV group. The authors concluded that prone positioning and allowance of spontaneous breathing during APRV had advantageous effects on gas exchange.
In 2004, the same investigators35 randomized 58 patients with ALI after stabilization to either APRV or pressure-controlled synchronized intermittent mandatory ventilation. There were no significant differences in the clinically important outcomes such as ventilator-free days, sedation days, need of hemodialysis, or intensive care unit-free days.
Dart et al,36 in a retrospective study of 46 trauma patients who were ventilated with APRV for 72 hours, found an improvement in the Pao2/Fio2 ratio and a decrement in peak airway pressure after APRV was started.
Table 2 summarizes the randomized clinical trials of APRV.33–35,37
CONCERNS ABOUT APRV
Overstretching. One of the major concerns when applying APRV is overstretching the lung parenchyma.26,38 It is important to recognize that, when choosing a P high setting, this variable is not the only determinant of the tidal volume. Spontaneous breathing causes the pleural pressure to become less positive. As a result, there is an increase in the transpulmonary pressure (pressure in alveoli minus pressure in the pleura). This augmentation of transpulmonary pressure will result in a higher tidal volume and the risk of overdistention and volume-induced lung injury.
Atelectrauma. As mentioned earlier, damage may occur when airways open and close with each tidal cycle. This is particularly worrisome when the end-expiratory pressure is below the lower inflection point, as some diseased alveolar units may collapse. In APRV, the airway pressure is released to zero. Even though the intentional auto-PEEP might maintain a certain end-expiratory pressure, this parameter is truly uncontrolled.39
If the patient cannot breath spontaneously. Another consideration is that many of the benefits of APRV are based on the spontaneous breathing component. Unfortunately, patients who need heavy sedation or neuromuscular paralysis with lack of spontaneous breathing efforts may lose the physiologic advantages of this mode.
Despite these limitations, APRV presents many attractive benefits as an alternative mode of mechanical ventilation in patients who do not respond to conventional modes.
Table 3 summarizes the advantages and disadvantages of each component of APRV.
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992; 18:319–321.
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–1786.
- Papadakos PJ, Lachmann B. The open lung concept of mechanical ventilation: the role of recruitment and stabilization. Crit Care Clin 2007; 23:241–250,
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Dreyfuss D, Saumon G, Hubmayr RD, editors. Ventilator-induced Lung Injury. New York: Taylor & Francis, 2006.
- Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006; 34:1311–1318.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Parsons PE, Eisner MD, Thompson BT, et al; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33:1–6.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354.
- Hemmila MR, Napolitano LM. Severe respiratory failure: advanced treatment options. Crit Care Med 2006; 34( suppl 9):S278–S290.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Martin LD, Wetzel RC. Optimal release time during airway pressure release ventilation in neonatal sheep. Crit Care Med 1994; 22:486–493.
- Frawley PM, Habashi NM. Airway pressure release ventilation: theory and practice. AACN Clin Issues 2001; 12:234–246.
- Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 159:1241–1248.
- Putensen C, Wrigge H. Clinical review: biphasic positive airway pressure and airway pressure release ventilation. Crit Care 2004; 8:492–497.
- Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33( suppl 3):S228–S240.
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41:242–255.
- Reber A, Nylund U, Hedenstierna G. Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia 1998; 53:1054–1061.
- Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158:1644–1655.
- Gattinoni L, Presenti A, Torresin A, et al. Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1986; 1:25–30.
- Hedenstierna G, Lichtwarck-Aschoff M. Interfacing spontaneous breathing and mechanical ventilation. New insights. Minerva Anestesiol 2006; 72:183–198.
- Neumann P, Wrigge H, Zinserling J, et al. Spontaneous breathing affects the spatial ventilation and perfusion distribution during mechanical ventilatory support. Crit Care Med 2005; 33:1090–1095.
- Räsänen J, Downs JB, Stock MC. Cardiovascular effects of conventional positive pressure ventilation and airway pressure release ventilation. Chest 1988; 93:911–915.
- Falkenhain SK, Reilley TE, Gregory JS. Improvement in cardiac output during airway pressure release ventilation. Crit Care Med 1992; 20:1358–1360.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med 2002; 28:1426–1433.
- Hering R, Viehöfer A, Zinserling J, et al. Effects of spontaneous breathing during airway pressure release ventilation on intestinal blood flow in experimental lung injury. Anesthesiology 2003; 99:1137–1144.
- Martin LD, Wetzel RC, Bilenki AL. Airway pressure release ventilation in a neonatal lamb model of acute lung injury. Crit Care Med 1991; 19:373–378.
- Garner W, Downs JB, Stock MC, Räsänen J. Airway pressure release ventilation (APRV). A human trial. Chest 1988; 94:779–781.
- Räsänen J, Cane RD, Downs JB, et al. Airway pressure release ventilation during acute lung injury: a prospective multicenter trial. Crit Care Med 1991; 19:1234–1241.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Varpula T, Jousela I, Niemi R, Takkunen O, Pettilä V. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand 2003; 47:516–524.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Dart BW, Maxwell RA, Richart CM, et al. Preliminary experience with airway pressure release ventilation in a trauma/surgical intensive care unit. J Trauma 2005; 59:71–76.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Dries DJ, Marini JJ. Airway pressure release ventilation. J Burn Care Res 2009; 30:929–936.
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992; 18:319–321.
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–1786.
- Papadakos PJ, Lachmann B. The open lung concept of mechanical ventilation: the role of recruitment and stabilization. Crit Care Clin 2007; 23:241–250,
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Dreyfuss D, Saumon G, Hubmayr RD, editors. Ventilator-induced Lung Injury. New York: Taylor & Francis, 2006.
- Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006; 34:1311–1318.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Parsons PE, Eisner MD, Thompson BT, et al; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33:1–6.
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354.
- Hemmila MR, Napolitano LM. Severe respiratory failure: advanced treatment options. Crit Care Med 2006; 34( suppl 9):S278–S290.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Martin LD, Wetzel RC. Optimal release time during airway pressure release ventilation in neonatal sheep. Crit Care Med 1994; 22:486–493.
- Frawley PM, Habashi NM. Airway pressure release ventilation: theory and practice. AACN Clin Issues 2001; 12:234–246.
- Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 159:1241–1248.
- Putensen C, Wrigge H. Clinical review: biphasic positive airway pressure and airway pressure release ventilation. Crit Care 2004; 8:492–497.
- Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33( suppl 3):S228–S240.
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41:242–255.
- Reber A, Nylund U, Hedenstierna G. Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia 1998; 53:1054–1061.
- Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158:1644–1655.
- Gattinoni L, Presenti A, Torresin A, et al. Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1986; 1:25–30.
- Hedenstierna G, Lichtwarck-Aschoff M. Interfacing spontaneous breathing and mechanical ventilation. New insights. Minerva Anestesiol 2006; 72:183–198.
- Neumann P, Wrigge H, Zinserling J, et al. Spontaneous breathing affects the spatial ventilation and perfusion distribution during mechanical ventilatory support. Crit Care Med 2005; 33:1090–1095.
- Räsänen J, Downs JB, Stock MC. Cardiovascular effects of conventional positive pressure ventilation and airway pressure release ventilation. Chest 1988; 93:911–915.
- Falkenhain SK, Reilley TE, Gregory JS. Improvement in cardiac output during airway pressure release ventilation. Crit Care Med 1992; 20:1358–1360.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med 2002; 28:1426–1433.
- Hering R, Viehöfer A, Zinserling J, et al. Effects of spontaneous breathing during airway pressure release ventilation on intestinal blood flow in experimental lung injury. Anesthesiology 2003; 99:1137–1144.
- Martin LD, Wetzel RC, Bilenki AL. Airway pressure release ventilation in a neonatal lamb model of acute lung injury. Crit Care Med 1991; 19:373–378.
- Garner W, Downs JB, Stock MC, Räsänen J. Airway pressure release ventilation (APRV). A human trial. Chest 1988; 94:779–781.
- Räsänen J, Cane RD, Downs JB, et al. Airway pressure release ventilation during acute lung injury: a prospective multicenter trial. Crit Care Med 1991; 19:1234–1241.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Varpula T, Jousela I, Niemi R, Takkunen O, Pettilä V. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand 2003; 47:516–524.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Dart BW, Maxwell RA, Richart CM, et al. Preliminary experience with airway pressure release ventilation in a trauma/surgical intensive care unit. J Trauma 2005; 59:71–76.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Dries DJ, Marini JJ. Airway pressure release ventilation. J Burn Care Res 2009; 30:929–936.
KEY POINTS
- The advantages and disadvantages of APRV are related to its two components: high mean airway pressure and spontaneous ventilation.
- Several studies show APRV to have physiologic benefits and to improve some measures of clinical outcome, such as oxygenation, use of sedation, hemodynamics, and respiratory mechanics.
- No study has reported that fewer patients die if they receive APRV compared with conventional protective ventilation.
- APRV is a promising mode, and further research is needed to strengthen support for its more widespread use.