User login
Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI
The use of gadolinium as a contrast agent in magnetic resonance imaging (MRI) in patients with impaired kidney function has come under scrutiny because of recent reports of a potential association between its use and nephrogenic systemic fibrosis (NSF).
This entity was first identified in the United States in 1997. Cowper et al1 in 2000 described 15 hemodialysis patients who developed thickening and hardening of the skin with brawny hyperpigmentation, papules, and subcutaneous nodules on the extremities.
This “new disease” was initially called “nephrogenic fibrosing dermopathy,” as it was exclusively seen in patients with renal impairment and was thought to affect only the skin and subcutaneous tissue. With growing evidence of the extent and pathogenicity of the fibrosis in visceral organs, the nomenclature was changed to NSF, to better reflect the systemic nature of the disease.
PRESENTATION: MILD TO DEVASTATING
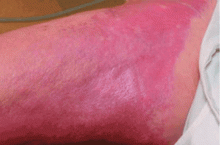
NSF has thus far been reported only in patients with renal impairment, most of whom were dialysis-dependent. It does not seem to be more common in one sex or the other, in any age range, or in any ethnic group. It can range in severity from mild to a devastating scleroderma-like systemic fibrosing disorder.
The heart, lungs, skeletal muscle, and diaphragm can also be involved, sometimes leading to serious complications and death.4–6
The disease is usually progressive and unremitting. Mendoza et al,7 in a review of 12 cases of NSF, reported that the disease had a progressive course in 6 patients, of whom 3 died within 2 years and 3 were ultimately confined to a wheelchair. More severe findings and rapid progression of the skin disease are associated with a poor prognosis.
Todd et al8 prospectively examined 186 dialysis patients to look for possible NSF. Of those with skin changes consistent with NSF, 48% died within 2 years, compared with 20% of those without these skin changes. Cardiovascular causes accounted for 58% of the deaths in patients with cutaneous changes of NSF and for 48% of the deaths in patients without these changes. Most of the excess deaths occurred within 6 months after the skin examination, suggesting an increased risk for early death in patients with skin changes suggestive of NSF.
DIAGNOSIS OF NSF IS CLINICAL
At presentation, NSF is frequently misdiagnosed and treated as cellulitis or edema. However, now that subspecialists—especially dermatologists, rheumatologists, and nephrologists—are becoming more aware of it, the correct diagnosis is being made earlier.
NSF should be suspected in any patient with underlying renal dysfunction—especially if on dialysis and if he or she has received a gadolinium contrast agent during MRI—who develops scleroderma-like cutaneous lesions affecting the distal extremities. Because most health care providers are still unfamiliar with this emerging disease, patients with renal impairment and suspected NSF should be referred to a rheumatologist or dermatologist to confirm the diagnosis, which is mainly entertained on a clinical basis. There is no laboratory biomarker for NSF.
A deep incisional skin biopsy may aid in the diagnosis. Due to the regional distribution of the disease, sampling error may occur, and repeat biopsy is warranted if the initial biopsy is nondiagnostic but the clinical picture suggests NSF.
Histopathologic examination typically shows lesions containing proliferation of dermal spindle cells, thick collagen bundles with surrounding clefts, and a variable amount of mucin and elastic fibers.2 A characteristic and almost pathognomonic staining profile is the immunohistochemical identification of CD34 reactivity in the fibroblast-like cells (Figure 3). Cells expressing CD34 are normally found in the umbilical cord, the bone marrow (as pluripotential hematopoietic stem cells), and in the vascular endothelium. How they come to be in the skin is still speculative, but their presence suggests that circulating fibrocytes migrate from the bone marrow and deposit in the skin and other organs.9,10
Pulmonary function testing can be done to rule out lung involvement and transthoracic two-dimensional echocardiography can be done to rule out possible cardiomyopathy if these conditions are suggested by examination at the time of diagnosis.7 Muscle biopsy is not necessary to determine the extent of systemic involvement, since the findings do not necessarily correlate with other systemic involvement.
DIFFERENTIAL DIAGNOSIS
An important diagnostic feature of NSF is that it spares the face, a finding derived from all reported and confirmed cases of NSF (Figure 2). In contrast, scleromyxedema, systemic scleroderma, and morphea often involve the face.
Scleromyxedema is often associated with monoclonal gammopathy (usually an immunoglobulin G lambda paraproteinemia) whereas NSF is not.
Scleroderma is supported by the findings of Raynaud’s phenomenon, antinuclear antibodies, and either anticentromere or anti-DNA topoisomerase I (Scl-70) antibodies, but the absence of these antibodies does not necessarily rule it out.
Eosinophilic fasciitis is diagnosed on the basis of histologic examination of a deep wedge skin biopsy specimen that includes fascia.
Other diagnoses that should be considered include amyloidosis and calciphylaxis.
ASSOCIATION WITH GADOLINIUM: WHAT IS THE EVIDENCE?
Case series
The association of gadolinium use with NSF has been described in several case reports and case series.
Grobner11 reported that administration of gadodiamide (Omniscan, a gadolinium compound) for MRI was associated with NSF in five patients on chronic hemodialysis who had end-stage renal disease. Their ages ranged from 43 to 74 years, and they had been on dialysis from 10 to 58 months. The time of onset of NSF ranged from 2 to 4 weeks after exposure to gadodiamide.
Marckmann et al12 reported that NSF developed in 13 (3.5%) of 370 patients with severe kidney disease who received gadodiamide. Five of the 13 patients had stage 5 (advanced) chronic kidney disease and were not yet on renal replacement therapy, 7 were on hemodialysis, and 1 was on peritoneal dialysis. The time of onset ranged from 2 to 75 days (median 25 days) after exposure.
Kuo et al13 similarly estimated the incidence of NSF at approximately 3% in patients with severe renal failure who receive intravenous gadolinium-based contrast material for MRI.
Broome et al14 reported that 12 patients developed NSF within 2 to 11 weeks after receiving gadodiamide. Eight of the 12 patients had end-stage renal disease and were on hemodialysis; the other 4 patients had acute kidney injury attributed to hepatorenal syndrome, and 3 of these 4 patients were on hemodialysis.
Khurana et al15 reported that 6 patients on hemodialysis developed NSF from 2 weeks to 2 months after receiving a dose of gadodiamide of between 0.11 and 0.36 mmol/kg. These doses are high, and the findings suggest an association between the gadolinium dose and NSF. The dose approved by the US Food and Drug Administration (FDA) is only 0.1 mmol/kg, and the use of gadolinium is approved only in MRI. However, higher doses (0.3–0.4 mmol/kg) are widely used in practice for better imaging quality in magnetic resonance angiography (MRA).
Deo et al16 reported 3 cases of NSF in 87 patients with end-stage renal disease who underwent 123 radiologic studies with gadolinium. No patient with end-stage renal disease who was not exposed to gadolinium developed NSF, and the association between exposure to gadolinium and the subsequent development of NSF was statistically significant (P = .006). The authors concluded that each gadolinium study presented a 2.4% risk of NSF in end-stage renal disease patients.
This retrospective study is flawed by not having been cross-sectional or case-controlled, since the other 84 patients who received gadolinium were not examined at all to establish the absence of NSF.
Case-control studies
More evidence of association of NSF with gadolinium exposure comes from other reports.
Physicians in St. Louis, MO,17 identified 33 cases of NSF and performed a case-control study, matching each of 19 of the patients (for whom data were available and who met their entry criteria) with 3 controls. They found that exposure to gadolinium was independently associated with the development of NSF.
Sadowski et al18 reported that 13 patients with biopsy-confirmed NSF all had been exposed to gadodiamide and one had been exposed to gadobenate (MultiHANCE) in addition to gadodiamide. All 13 patients had renal insufficiency, with an estimated glomerular filtration rate (GFR) less than 60 mL/minute/1.73 m2. The investigators compared this group with a control group of patients with renal insufficiency who did not develop NSF. The NSF group had more proinflammatory events (P < .001) and more gadolinium-contrast-enhanced MRI examinations per patient (P = .002) than the control group.
Marckmann et al19 compared 19 patients who had histologically proven cases of NSF and 19 sex- and age-matched controls; all 38 patients had chronic kidney disease and had been exposed to gadolinium. Patients with NSF had received higher cumulative doses of gadodiamide and higher doses of erythropoietin and had higher serum concentrations of ionized calcium and phosphate than did their controls, as did patients with severe NSF compared with those with nonsevere NSF.
Comment. All the above reports are limited by their study design and suffer from recognition bias because not all of the patients with severe renal insufficiency who were exposed to gadolinium were examined for possible asymptomatic skin changes that might be characteristic of NSF. Therefore, it is impossible to be certain that all of the patients classified as not having NSF truly did not have it or did not subsequently develop it. Furthermore, the reports lacked standardized diagnostic criteria. Hence, the real prevalence and incidence of NSF are difficult to determine.
A cross-sectional study
As mentioned above, Todd et al8 examined 186 dialysis patients for cutaneous changes of NSF (using a scoring system based on hyper-pigmentation, hardening, and tethering of skin on the extremities). Patients who had been exposed to gadolinium had a higher risk of developing these skin changes than did nonexposed patients (odds ratio 14.7, 95% confidence interval 1.9–117.0). More importantly, the investigators found cutaneous changes of NSF in 25 (13%) of the 186 patients, 4 of whom had prior skin biopsies available for review, each revealing the histologic changes of NSF. This study suggests that NSF may be more prevalent than previously thought.
Is kidney dysfunction always present?
All the reported patients with NSF had underlying renal impairment. The renal dysfunction ranged from acute kidney injury to advanced chronic kidney disease (estimated GFR < 30 mL/minute/1.73 m2) and end-stage renal disease on renal replacement therapy, ie, hemodialysis or peritoneal dialysis. The incidence of NSF does not seem to be related to the cause of the underlying kidney disease.
What other diseases or comorbidities can be associated with NSF?
It is still unclear why not every patient with advanced renal failure develops NSF after exposure to gadolinium.
A variety of complex diseases and conditions have been reported to be associated with NSF, with no clear-cut evidence of causality or trigger. These include hypercoagulability states, thrombotic events, surgical procedures (especially those with reconstructive vascular components), calciphylaxis, kidney transplantation, hepatic disease (hepatorenal syndrome, liver transplantation, and hepatitis B and C), idiopathic pulmonary fibrosis, systemic lupus erythematosus, hypothyroidism, elevated serum ionized calcium or serum phosphate, hyperparathyroidism, and metabolic acidosis. A possible explanation is that most of these conditions are associated with an increased use of MRI or MRA testing (eg, in the workup for kidney or liver transplantation).
Many drugs have also been reported to be associated with NSF, including high-dose erythropoietin,20 sevelamer (Renagel),21 and, conversely, lack of angiotensin-converting enzyme inhibitor therapy,22 but none of these findings has been reproduced to date.
GADOLINIUM CHARACTERISTICS AND PHARMACOKINETICS
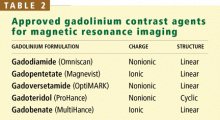
Gadolinium is a rare-earth lanthanide metallic element (atomic number 64) that is used in MRI and MRA because of its paramagnetic properties that enhance the quality of imaging. Its ionic form (Gd3+) is highly toxic if injected intravenously, so it is typically bound to a “chelate” to decrease its toxicity.23 The chelate stabilizes Gd3+ and thereby prevents its dissociation in vivo. These Gd-chelates can be classified (Table 2) according to their charge (ionic vs nonionic) and their structure (linear vs cyclic).
Most of the reported cases of NSF have been in patients who received gadodiamide, a nonionic, linear agent. Why gadodiamide has the highest rates of association with NSF is still unclear; perhaps it is simply the most widely used agent. Also, linear Gd compounds may be less stable and more likely to dissociate in vivo. The updated FDA Public Health Advisory in May 2007 warned against the use of all gadolinium-containing contrast agents for MRI, not just gadodiamide.
After intravenous injection, Gd-chelate equilibrates rapidly (within 2 hours) in the extracellular space. Very little of it enters into cells or binds to proteins. It is eliminated unchanged in the glomerular filtrate with no tubular secretion. In a study by Joffe et al,24 the elimination half-life of gadodiamide in patients with severely reduced renal function was considerably longer than in healthy volunteers (34.3 hours ± 22.9 vs 1.3 hours ± 0.25).
Since gadolinium compounds are not protein-bound and have a limited volume of distribution, they are typically removed by hemodialysis. Joffe et al found that an average of 65% of the gadodiamide was removed in a single hemodialysis session. However, they did not describe the specific features of the hemodialysis session, and it took four hemodialysis treatments to remove 99% of a single dose of gadolinium.24 A dialysis membrane with high permeability (large pores) seems to increase the clearance of the Gd-chelate during hemodialysis.25
Peritoneal dialysis may not remove gadolinium as effectively: Joffe et al24 reported that after 22 days of continuous ambulatory peritoneal dialysis, only 69% of the total amount of gadodiamide had been excreted, suggesting a very low peritoneal clearance.
SPECULATIVE PATHOGENESIS
Although a causal relationship between gadolinium use in patients with renal dysfunction and NSF has not been definitively established, the data derived from case reports assuredly raise this suspicion. Furthermore, on biopsy, gadolinium can be found in the skin of patients with NSF, adding evidence of causality.26–28
The mechanism by which Gd3+ might trigger NSF is still not understood. A plausible speculation is that if renal function is reduced, the half-life of the Gd-chelate molecule is significantly increased, as is the chance of Gd3+ dissociating from its chelate, leading to increased tissue exposure. Vascular trauma and endothelial dysfunction may allow free Gd3+ to enter tissues more easily, where macrophages phagocytose the metal, produce local profibrotic cytokines, and send out signals that recruit circulating fibrocytes to the tissues. Once in tissues, circulating fibrocytes induce a fibrosing process that is indistinguishable from normal scar formation.29
TREATMENTS LACK DATA
There is no consistently successful treatment for NSF.
In isolated reports, successful kidney transplantation slowed the skin fibrosis, but these findings need to be confirmed.30,31 Data from case reports should be interpreted very cautiously, as they are by nature sporadic and anecdotal. Moreover most of the reports of NSF were published on Web sites or as editorials and did not undergo exhaustive peer review. Because the evidence is weak, kidney transplantation should not be recommended as a treatment for NSF.
Oral steroids, plasmapheresis, extracorporeal photopheresis, thalidomide, topical ultraviolet-A therapy, and other treatments have yielded very conflicting results, with only anecdotal improvement of symptoms. In a recent case report,32 the use of intravenous sodium thiosulfate in addition to aggressive physical therapy provided some benefit by reducing the pain and improving the skin lesions.
Because of the lack of strong evidence of efficacy, we cannot advocate the use of any of these treatments until larger clinical trial results are available. Aggressive physical therapy along with appropriate pain control may have benefits and should be offered to all patients suffering from NSF.
Avoid gadolinium exposure in patients with renal insufficiency
The FDA33 recently asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents (Magnevist, MultiHance, Omniscan, Opti-MARK, ProHance), due to risk of NSF in patients with acute or chronic severe renal insufficiency (GFR < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
For the time being, gadolinium should be contraindicated in patients with acute kidney injury and chronic kidney disease stages 4 and 5 and in those who are on renal replacement therapy (either hemodialysis or peritoneal dialysis). If an MRI study with gadolinium-based contrast is absolutely required in a patient with end-stage renal disease or advanced chronic kidney disease, an agent other than gadodiamide should be used in the lowest possible dose.
Will hemodialysis prevent NSF?
In a patient who is already on hemodialysis, it seems prudent to perform hemodialysis soon after gadolinium exposure and again the day after exposure to increase gadolinium elimination. However, to date, there are no data to support the theory that doing this will prevent NSF.
Because peritoneal dialysis has been reported to clear gadolinium poorly, use of gadolinium is contraindicated. If gadolinium is absolutely needed, either more-aggressive peritoneal dialysis (keeping the abdomen “wet”) or temporary hemodialysis may be considered.
For patients with advanced chronic kidney disease who are not yet on renal replacement therapy, the use of gadolinium is contraindicated, and hemodialysis should not be empirically recommended after gadolinium exposure because we have no evidence to support its utility and because hemodialysis may cause harm.
Nephrology consultation should be considered before any gadolinium use in a patient with impaired renal function, whether acute or chronic.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18:614–617.
- Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003; 15:785–790.
- Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003; 139:903–906.
- Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol 2006; 54:S31–S34.
- Jimenez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum 2004; 50:2660–2666.
- Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 2006; 35:238–249.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Cowper SE, Bucala R, Leboit PE. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis—setting the record straight. Semin Arthritis Rheum 2006; 35:208–210.
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004; 36:598–606.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21:1104–1108.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17:2359–2362.
- Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007; 242:647–649.
- Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007; 188:586–592.
- Khurana A, Runge VM, Narayanan M, Greene JF, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol 2007; 42:139–145.
- Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007; 2:264–267.
- US Centers for Disease Control and Prevention (CDC). Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002–2006. MMWR Morb Mortal Wkly Rep 2007; 56:137–141.
- Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007; 243:148–157.
- Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 2007 May 4; e-pub ahead of print.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Jain SM, Wesson S, Hassanein A, et al. Nephrogenic fibrosing dermopathy in pediatric patients. Pediatr Nephrol 2004; 19:467–470.
- Fazeli A, Lio PA, Liu V. Nephrogenic fibrosing dermopathy: are ACE inhibitors the missing link? (Letter). Arch Dermatol 2004; 140:1401.
- Bellin MF. MR contrast agents, the old and the new. Eur J Radiol 2006; 60:314–323.
- Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 1998; 5:491–502.
- Ueda J, Furukawa T, Higashino K, et al. Permeability of iodinated and MR contrast media through two types of hemodialysis membrane. Eur J Radiol 1999; 31:76–80.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis, J Am Acad Dermatol 2007; 56:710–712.
- Perazella MA. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: is there a link? Clin J Am Soc Nephrol 2007; 2:200–202.
- Cowper SE. Nephrogenic systemic fibrosis: The nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis 2005; 46:763–765.
- Jan F, Segal JM, Dyer J, LeBoit P, Siegfried E, Frieden IJ. Nephrogenic fibrosing dermopathy: two pediatric cases. J Pediatr 2003; 143:678–681.
- Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure—role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol 2007; 2:258–263.
- US Food and Drug Administration. Accessed 01/03/08. http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm.
The use of gadolinium as a contrast agent in magnetic resonance imaging (MRI) in patients with impaired kidney function has come under scrutiny because of recent reports of a potential association between its use and nephrogenic systemic fibrosis (NSF).
This entity was first identified in the United States in 1997. Cowper et al1 in 2000 described 15 hemodialysis patients who developed thickening and hardening of the skin with brawny hyperpigmentation, papules, and subcutaneous nodules on the extremities.
This “new disease” was initially called “nephrogenic fibrosing dermopathy,” as it was exclusively seen in patients with renal impairment and was thought to affect only the skin and subcutaneous tissue. With growing evidence of the extent and pathogenicity of the fibrosis in visceral organs, the nomenclature was changed to NSF, to better reflect the systemic nature of the disease.
PRESENTATION: MILD TO DEVASTATING
NSF has thus far been reported only in patients with renal impairment, most of whom were dialysis-dependent. It does not seem to be more common in one sex or the other, in any age range, or in any ethnic group. It can range in severity from mild to a devastating scleroderma-like systemic fibrosing disorder.
The heart, lungs, skeletal muscle, and diaphragm can also be involved, sometimes leading to serious complications and death.4–6
The disease is usually progressive and unremitting. Mendoza et al,7 in a review of 12 cases of NSF, reported that the disease had a progressive course in 6 patients, of whom 3 died within 2 years and 3 were ultimately confined to a wheelchair. More severe findings and rapid progression of the skin disease are associated with a poor prognosis.
Todd et al8 prospectively examined 186 dialysis patients to look for possible NSF. Of those with skin changes consistent with NSF, 48% died within 2 years, compared with 20% of those without these skin changes. Cardiovascular causes accounted for 58% of the deaths in patients with cutaneous changes of NSF and for 48% of the deaths in patients without these changes. Most of the excess deaths occurred within 6 months after the skin examination, suggesting an increased risk for early death in patients with skin changes suggestive of NSF.
DIAGNOSIS OF NSF IS CLINICAL
At presentation, NSF is frequently misdiagnosed and treated as cellulitis or edema. However, now that subspecialists—especially dermatologists, rheumatologists, and nephrologists—are becoming more aware of it, the correct diagnosis is being made earlier.
NSF should be suspected in any patient with underlying renal dysfunction—especially if on dialysis and if he or she has received a gadolinium contrast agent during MRI—who develops scleroderma-like cutaneous lesions affecting the distal extremities. Because most health care providers are still unfamiliar with this emerging disease, patients with renal impairment and suspected NSF should be referred to a rheumatologist or dermatologist to confirm the diagnosis, which is mainly entertained on a clinical basis. There is no laboratory biomarker for NSF.
A deep incisional skin biopsy may aid in the diagnosis. Due to the regional distribution of the disease, sampling error may occur, and repeat biopsy is warranted if the initial biopsy is nondiagnostic but the clinical picture suggests NSF.
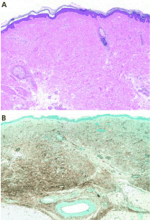
Histopathologic examination typically shows lesions containing proliferation of dermal spindle cells, thick collagen bundles with surrounding clefts, and a variable amount of mucin and elastic fibers.2 A characteristic and almost pathognomonic staining profile is the immunohistochemical identification of CD34 reactivity in the fibroblast-like cells (Figure 3). Cells expressing CD34 are normally found in the umbilical cord, the bone marrow (as pluripotential hematopoietic stem cells), and in the vascular endothelium. How they come to be in the skin is still speculative, but their presence suggests that circulating fibrocytes migrate from the bone marrow and deposit in the skin and other organs.9,10
Pulmonary function testing can be done to rule out lung involvement and transthoracic two-dimensional echocardiography can be done to rule out possible cardiomyopathy if these conditions are suggested by examination at the time of diagnosis.7 Muscle biopsy is not necessary to determine the extent of systemic involvement, since the findings do not necessarily correlate with other systemic involvement.
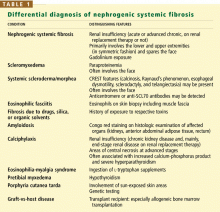
DIFFERENTIAL DIAGNOSIS
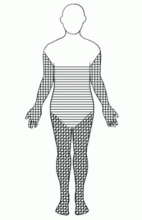
An important diagnostic feature of NSF is that it spares the face, a finding derived from all reported and confirmed cases of NSF (Figure 2). In contrast, scleromyxedema, systemic scleroderma, and morphea often involve the face.
Scleromyxedema is often associated with monoclonal gammopathy (usually an immunoglobulin G lambda paraproteinemia) whereas NSF is not.
Scleroderma is supported by the findings of Raynaud’s phenomenon, antinuclear antibodies, and either anticentromere or anti-DNA topoisomerase I (Scl-70) antibodies, but the absence of these antibodies does not necessarily rule it out.
Eosinophilic fasciitis is diagnosed on the basis of histologic examination of a deep wedge skin biopsy specimen that includes fascia.
Other diagnoses that should be considered include amyloidosis and calciphylaxis.
ASSOCIATION WITH GADOLINIUM: WHAT IS THE EVIDENCE?
Case series
The association of gadolinium use with NSF has been described in several case reports and case series.
Grobner11 reported that administration of gadodiamide (Omniscan, a gadolinium compound) for MRI was associated with NSF in five patients on chronic hemodialysis who had end-stage renal disease. Their ages ranged from 43 to 74 years, and they had been on dialysis from 10 to 58 months. The time of onset of NSF ranged from 2 to 4 weeks after exposure to gadodiamide.
Marckmann et al12 reported that NSF developed in 13 (3.5%) of 370 patients with severe kidney disease who received gadodiamide. Five of the 13 patients had stage 5 (advanced) chronic kidney disease and were not yet on renal replacement therapy, 7 were on hemodialysis, and 1 was on peritoneal dialysis. The time of onset ranged from 2 to 75 days (median 25 days) after exposure.
Kuo et al13 similarly estimated the incidence of NSF at approximately 3% in patients with severe renal failure who receive intravenous gadolinium-based contrast material for MRI.
Broome et al14 reported that 12 patients developed NSF within 2 to 11 weeks after receiving gadodiamide. Eight of the 12 patients had end-stage renal disease and were on hemodialysis; the other 4 patients had acute kidney injury attributed to hepatorenal syndrome, and 3 of these 4 patients were on hemodialysis.
Khurana et al15 reported that 6 patients on hemodialysis developed NSF from 2 weeks to 2 months after receiving a dose of gadodiamide of between 0.11 and 0.36 mmol/kg. These doses are high, and the findings suggest an association between the gadolinium dose and NSF. The dose approved by the US Food and Drug Administration (FDA) is only 0.1 mmol/kg, and the use of gadolinium is approved only in MRI. However, higher doses (0.3–0.4 mmol/kg) are widely used in practice for better imaging quality in magnetic resonance angiography (MRA).
Deo et al16 reported 3 cases of NSF in 87 patients with end-stage renal disease who underwent 123 radiologic studies with gadolinium. No patient with end-stage renal disease who was not exposed to gadolinium developed NSF, and the association between exposure to gadolinium and the subsequent development of NSF was statistically significant (P = .006). The authors concluded that each gadolinium study presented a 2.4% risk of NSF in end-stage renal disease patients.
This retrospective study is flawed by not having been cross-sectional or case-controlled, since the other 84 patients who received gadolinium were not examined at all to establish the absence of NSF.
Case-control studies
More evidence of association of NSF with gadolinium exposure comes from other reports.
Physicians in St. Louis, MO,17 identified 33 cases of NSF and performed a case-control study, matching each of 19 of the patients (for whom data were available and who met their entry criteria) with 3 controls. They found that exposure to gadolinium was independently associated with the development of NSF.
Sadowski et al18 reported that 13 patients with biopsy-confirmed NSF all had been exposed to gadodiamide and one had been exposed to gadobenate (MultiHANCE) in addition to gadodiamide. All 13 patients had renal insufficiency, with an estimated glomerular filtration rate (GFR) less than 60 mL/minute/1.73 m2. The investigators compared this group with a control group of patients with renal insufficiency who did not develop NSF. The NSF group had more proinflammatory events (P < .001) and more gadolinium-contrast-enhanced MRI examinations per patient (P = .002) than the control group.
Marckmann et al19 compared 19 patients who had histologically proven cases of NSF and 19 sex- and age-matched controls; all 38 patients had chronic kidney disease and had been exposed to gadolinium. Patients with NSF had received higher cumulative doses of gadodiamide and higher doses of erythropoietin and had higher serum concentrations of ionized calcium and phosphate than did their controls, as did patients with severe NSF compared with those with nonsevere NSF.
Comment. All the above reports are limited by their study design and suffer from recognition bias because not all of the patients with severe renal insufficiency who were exposed to gadolinium were examined for possible asymptomatic skin changes that might be characteristic of NSF. Therefore, it is impossible to be certain that all of the patients classified as not having NSF truly did not have it or did not subsequently develop it. Furthermore, the reports lacked standardized diagnostic criteria. Hence, the real prevalence and incidence of NSF are difficult to determine.
A cross-sectional study
As mentioned above, Todd et al8 examined 186 dialysis patients for cutaneous changes of NSF (using a scoring system based on hyper-pigmentation, hardening, and tethering of skin on the extremities). Patients who had been exposed to gadolinium had a higher risk of developing these skin changes than did nonexposed patients (odds ratio 14.7, 95% confidence interval 1.9–117.0). More importantly, the investigators found cutaneous changes of NSF in 25 (13%) of the 186 patients, 4 of whom had prior skin biopsies available for review, each revealing the histologic changes of NSF. This study suggests that NSF may be more prevalent than previously thought.
Is kidney dysfunction always present?
All the reported patients with NSF had underlying renal impairment. The renal dysfunction ranged from acute kidney injury to advanced chronic kidney disease (estimated GFR < 30 mL/minute/1.73 m2) and end-stage renal disease on renal replacement therapy, ie, hemodialysis or peritoneal dialysis. The incidence of NSF does not seem to be related to the cause of the underlying kidney disease.
What other diseases or comorbidities can be associated with NSF?
It is still unclear why not every patient with advanced renal failure develops NSF after exposure to gadolinium.
A variety of complex diseases and conditions have been reported to be associated with NSF, with no clear-cut evidence of causality or trigger. These include hypercoagulability states, thrombotic events, surgical procedures (especially those with reconstructive vascular components), calciphylaxis, kidney transplantation, hepatic disease (hepatorenal syndrome, liver transplantation, and hepatitis B and C), idiopathic pulmonary fibrosis, systemic lupus erythematosus, hypothyroidism, elevated serum ionized calcium or serum phosphate, hyperparathyroidism, and metabolic acidosis. A possible explanation is that most of these conditions are associated with an increased use of MRI or MRA testing (eg, in the workup for kidney or liver transplantation).
Many drugs have also been reported to be associated with NSF, including high-dose erythropoietin,20 sevelamer (Renagel),21 and, conversely, lack of angiotensin-converting enzyme inhibitor therapy,22 but none of these findings has been reproduced to date.
GADOLINIUM CHARACTERISTICS AND PHARMACOKINETICS
Gadolinium is a rare-earth lanthanide metallic element (atomic number 64) that is used in MRI and MRA because of its paramagnetic properties that enhance the quality of imaging. Its ionic form (Gd3+) is highly toxic if injected intravenously, so it is typically bound to a “chelate” to decrease its toxicity.23 The chelate stabilizes Gd3+ and thereby prevents its dissociation in vivo. These Gd-chelates can be classified (Table 2) according to their charge (ionic vs nonionic) and their structure (linear vs cyclic).
Most of the reported cases of NSF have been in patients who received gadodiamide, a nonionic, linear agent. Why gadodiamide has the highest rates of association with NSF is still unclear; perhaps it is simply the most widely used agent. Also, linear Gd compounds may be less stable and more likely to dissociate in vivo. The updated FDA Public Health Advisory in May 2007 warned against the use of all gadolinium-containing contrast agents for MRI, not just gadodiamide.
After intravenous injection, Gd-chelate equilibrates rapidly (within 2 hours) in the extracellular space. Very little of it enters into cells or binds to proteins. It is eliminated unchanged in the glomerular filtrate with no tubular secretion. In a study by Joffe et al,24 the elimination half-life of gadodiamide in patients with severely reduced renal function was considerably longer than in healthy volunteers (34.3 hours ± 22.9 vs 1.3 hours ± 0.25).
Since gadolinium compounds are not protein-bound and have a limited volume of distribution, they are typically removed by hemodialysis. Joffe et al found that an average of 65% of the gadodiamide was removed in a single hemodialysis session. However, they did not describe the specific features of the hemodialysis session, and it took four hemodialysis treatments to remove 99% of a single dose of gadolinium.24 A dialysis membrane with high permeability (large pores) seems to increase the clearance of the Gd-chelate during hemodialysis.25
Peritoneal dialysis may not remove gadolinium as effectively: Joffe et al24 reported that after 22 days of continuous ambulatory peritoneal dialysis, only 69% of the total amount of gadodiamide had been excreted, suggesting a very low peritoneal clearance.
SPECULATIVE PATHOGENESIS
Although a causal relationship between gadolinium use in patients with renal dysfunction and NSF has not been definitively established, the data derived from case reports assuredly raise this suspicion. Furthermore, on biopsy, gadolinium can be found in the skin of patients with NSF, adding evidence of causality.26–28
The mechanism by which Gd3+ might trigger NSF is still not understood. A plausible speculation is that if renal function is reduced, the half-life of the Gd-chelate molecule is significantly increased, as is the chance of Gd3+ dissociating from its chelate, leading to increased tissue exposure. Vascular trauma and endothelial dysfunction may allow free Gd3+ to enter tissues more easily, where macrophages phagocytose the metal, produce local profibrotic cytokines, and send out signals that recruit circulating fibrocytes to the tissues. Once in tissues, circulating fibrocytes induce a fibrosing process that is indistinguishable from normal scar formation.29
TREATMENTS LACK DATA
There is no consistently successful treatment for NSF.
In isolated reports, successful kidney transplantation slowed the skin fibrosis, but these findings need to be confirmed.30,31 Data from case reports should be interpreted very cautiously, as they are by nature sporadic and anecdotal. Moreover most of the reports of NSF were published on Web sites or as editorials and did not undergo exhaustive peer review. Because the evidence is weak, kidney transplantation should not be recommended as a treatment for NSF.
Oral steroids, plasmapheresis, extracorporeal photopheresis, thalidomide, topical ultraviolet-A therapy, and other treatments have yielded very conflicting results, with only anecdotal improvement of symptoms. In a recent case report,32 the use of intravenous sodium thiosulfate in addition to aggressive physical therapy provided some benefit by reducing the pain and improving the skin lesions.
Because of the lack of strong evidence of efficacy, we cannot advocate the use of any of these treatments until larger clinical trial results are available. Aggressive physical therapy along with appropriate pain control may have benefits and should be offered to all patients suffering from NSF.
Avoid gadolinium exposure in patients with renal insufficiency
The FDA33 recently asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents (Magnevist, MultiHance, Omniscan, Opti-MARK, ProHance), due to risk of NSF in patients with acute or chronic severe renal insufficiency (GFR < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
For the time being, gadolinium should be contraindicated in patients with acute kidney injury and chronic kidney disease stages 4 and 5 and in those who are on renal replacement therapy (either hemodialysis or peritoneal dialysis). If an MRI study with gadolinium-based contrast is absolutely required in a patient with end-stage renal disease or advanced chronic kidney disease, an agent other than gadodiamide should be used in the lowest possible dose.
Will hemodialysis prevent NSF?
In a patient who is already on hemodialysis, it seems prudent to perform hemodialysis soon after gadolinium exposure and again the day after exposure to increase gadolinium elimination. However, to date, there are no data to support the theory that doing this will prevent NSF.
Because peritoneal dialysis has been reported to clear gadolinium poorly, use of gadolinium is contraindicated. If gadolinium is absolutely needed, either more-aggressive peritoneal dialysis (keeping the abdomen “wet”) or temporary hemodialysis may be considered.
For patients with advanced chronic kidney disease who are not yet on renal replacement therapy, the use of gadolinium is contraindicated, and hemodialysis should not be empirically recommended after gadolinium exposure because we have no evidence to support its utility and because hemodialysis may cause harm.
Nephrology consultation should be considered before any gadolinium use in a patient with impaired renal function, whether acute or chronic.
The use of gadolinium as a contrast agent in magnetic resonance imaging (MRI) in patients with impaired kidney function has come under scrutiny because of recent reports of a potential association between its use and nephrogenic systemic fibrosis (NSF).
This entity was first identified in the United States in 1997. Cowper et al1 in 2000 described 15 hemodialysis patients who developed thickening and hardening of the skin with brawny hyperpigmentation, papules, and subcutaneous nodules on the extremities.
This “new disease” was initially called “nephrogenic fibrosing dermopathy,” as it was exclusively seen in patients with renal impairment and was thought to affect only the skin and subcutaneous tissue. With growing evidence of the extent and pathogenicity of the fibrosis in visceral organs, the nomenclature was changed to NSF, to better reflect the systemic nature of the disease.
PRESENTATION: MILD TO DEVASTATING
NSF has thus far been reported only in patients with renal impairment, most of whom were dialysis-dependent. It does not seem to be more common in one sex or the other, in any age range, or in any ethnic group. It can range in severity from mild to a devastating scleroderma-like systemic fibrosing disorder.
The heart, lungs, skeletal muscle, and diaphragm can also be involved, sometimes leading to serious complications and death.4–6
The disease is usually progressive and unremitting. Mendoza et al,7 in a review of 12 cases of NSF, reported that the disease had a progressive course in 6 patients, of whom 3 died within 2 years and 3 were ultimately confined to a wheelchair. More severe findings and rapid progression of the skin disease are associated with a poor prognosis.
Todd et al8 prospectively examined 186 dialysis patients to look for possible NSF. Of those with skin changes consistent with NSF, 48% died within 2 years, compared with 20% of those without these skin changes. Cardiovascular causes accounted for 58% of the deaths in patients with cutaneous changes of NSF and for 48% of the deaths in patients without these changes. Most of the excess deaths occurred within 6 months after the skin examination, suggesting an increased risk for early death in patients with skin changes suggestive of NSF.
DIAGNOSIS OF NSF IS CLINICAL
At presentation, NSF is frequently misdiagnosed and treated as cellulitis or edema. However, now that subspecialists—especially dermatologists, rheumatologists, and nephrologists—are becoming more aware of it, the correct diagnosis is being made earlier.
NSF should be suspected in any patient with underlying renal dysfunction—especially if on dialysis and if he or she has received a gadolinium contrast agent during MRI—who develops scleroderma-like cutaneous lesions affecting the distal extremities. Because most health care providers are still unfamiliar with this emerging disease, patients with renal impairment and suspected NSF should be referred to a rheumatologist or dermatologist to confirm the diagnosis, which is mainly entertained on a clinical basis. There is no laboratory biomarker for NSF.
A deep incisional skin biopsy may aid in the diagnosis. Due to the regional distribution of the disease, sampling error may occur, and repeat biopsy is warranted if the initial biopsy is nondiagnostic but the clinical picture suggests NSF.
Histopathologic examination typically shows lesions containing proliferation of dermal spindle cells, thick collagen bundles with surrounding clefts, and a variable amount of mucin and elastic fibers.2 A characteristic and almost pathognomonic staining profile is the immunohistochemical identification of CD34 reactivity in the fibroblast-like cells (Figure 3). Cells expressing CD34 are normally found in the umbilical cord, the bone marrow (as pluripotential hematopoietic stem cells), and in the vascular endothelium. How they come to be in the skin is still speculative, but their presence suggests that circulating fibrocytes migrate from the bone marrow and deposit in the skin and other organs.9,10
Pulmonary function testing can be done to rule out lung involvement and transthoracic two-dimensional echocardiography can be done to rule out possible cardiomyopathy if these conditions are suggested by examination at the time of diagnosis.7 Muscle biopsy is not necessary to determine the extent of systemic involvement, since the findings do not necessarily correlate with other systemic involvement.
DIFFERENTIAL DIAGNOSIS
An important diagnostic feature of NSF is that it spares the face, a finding derived from all reported and confirmed cases of NSF (Figure 2). In contrast, scleromyxedema, systemic scleroderma, and morphea often involve the face.
Scleromyxedema is often associated with monoclonal gammopathy (usually an immunoglobulin G lambda paraproteinemia) whereas NSF is not.
Scleroderma is supported by the findings of Raynaud’s phenomenon, antinuclear antibodies, and either anticentromere or anti-DNA topoisomerase I (Scl-70) antibodies, but the absence of these antibodies does not necessarily rule it out.
Eosinophilic fasciitis is diagnosed on the basis of histologic examination of a deep wedge skin biopsy specimen that includes fascia.
Other diagnoses that should be considered include amyloidosis and calciphylaxis.
ASSOCIATION WITH GADOLINIUM: WHAT IS THE EVIDENCE?
Case series
The association of gadolinium use with NSF has been described in several case reports and case series.
Grobner11 reported that administration of gadodiamide (Omniscan, a gadolinium compound) for MRI was associated with NSF in five patients on chronic hemodialysis who had end-stage renal disease. Their ages ranged from 43 to 74 years, and they had been on dialysis from 10 to 58 months. The time of onset of NSF ranged from 2 to 4 weeks after exposure to gadodiamide.
Marckmann et al12 reported that NSF developed in 13 (3.5%) of 370 patients with severe kidney disease who received gadodiamide. Five of the 13 patients had stage 5 (advanced) chronic kidney disease and were not yet on renal replacement therapy, 7 were on hemodialysis, and 1 was on peritoneal dialysis. The time of onset ranged from 2 to 75 days (median 25 days) after exposure.
Kuo et al13 similarly estimated the incidence of NSF at approximately 3% in patients with severe renal failure who receive intravenous gadolinium-based contrast material for MRI.
Broome et al14 reported that 12 patients developed NSF within 2 to 11 weeks after receiving gadodiamide. Eight of the 12 patients had end-stage renal disease and were on hemodialysis; the other 4 patients had acute kidney injury attributed to hepatorenal syndrome, and 3 of these 4 patients were on hemodialysis.
Khurana et al15 reported that 6 patients on hemodialysis developed NSF from 2 weeks to 2 months after receiving a dose of gadodiamide of between 0.11 and 0.36 mmol/kg. These doses are high, and the findings suggest an association between the gadolinium dose and NSF. The dose approved by the US Food and Drug Administration (FDA) is only 0.1 mmol/kg, and the use of gadolinium is approved only in MRI. However, higher doses (0.3–0.4 mmol/kg) are widely used in practice for better imaging quality in magnetic resonance angiography (MRA).
Deo et al16 reported 3 cases of NSF in 87 patients with end-stage renal disease who underwent 123 radiologic studies with gadolinium. No patient with end-stage renal disease who was not exposed to gadolinium developed NSF, and the association between exposure to gadolinium and the subsequent development of NSF was statistically significant (P = .006). The authors concluded that each gadolinium study presented a 2.4% risk of NSF in end-stage renal disease patients.
This retrospective study is flawed by not having been cross-sectional or case-controlled, since the other 84 patients who received gadolinium were not examined at all to establish the absence of NSF.
Case-control studies
More evidence of association of NSF with gadolinium exposure comes from other reports.
Physicians in St. Louis, MO,17 identified 33 cases of NSF and performed a case-control study, matching each of 19 of the patients (for whom data were available and who met their entry criteria) with 3 controls. They found that exposure to gadolinium was independently associated with the development of NSF.
Sadowski et al18 reported that 13 patients with biopsy-confirmed NSF all had been exposed to gadodiamide and one had been exposed to gadobenate (MultiHANCE) in addition to gadodiamide. All 13 patients had renal insufficiency, with an estimated glomerular filtration rate (GFR) less than 60 mL/minute/1.73 m2. The investigators compared this group with a control group of patients with renal insufficiency who did not develop NSF. The NSF group had more proinflammatory events (P < .001) and more gadolinium-contrast-enhanced MRI examinations per patient (P = .002) than the control group.
Marckmann et al19 compared 19 patients who had histologically proven cases of NSF and 19 sex- and age-matched controls; all 38 patients had chronic kidney disease and had been exposed to gadolinium. Patients with NSF had received higher cumulative doses of gadodiamide and higher doses of erythropoietin and had higher serum concentrations of ionized calcium and phosphate than did their controls, as did patients with severe NSF compared with those with nonsevere NSF.
Comment. All the above reports are limited by their study design and suffer from recognition bias because not all of the patients with severe renal insufficiency who were exposed to gadolinium were examined for possible asymptomatic skin changes that might be characteristic of NSF. Therefore, it is impossible to be certain that all of the patients classified as not having NSF truly did not have it or did not subsequently develop it. Furthermore, the reports lacked standardized diagnostic criteria. Hence, the real prevalence and incidence of NSF are difficult to determine.
A cross-sectional study
As mentioned above, Todd et al8 examined 186 dialysis patients for cutaneous changes of NSF (using a scoring system based on hyper-pigmentation, hardening, and tethering of skin on the extremities). Patients who had been exposed to gadolinium had a higher risk of developing these skin changes than did nonexposed patients (odds ratio 14.7, 95% confidence interval 1.9–117.0). More importantly, the investigators found cutaneous changes of NSF in 25 (13%) of the 186 patients, 4 of whom had prior skin biopsies available for review, each revealing the histologic changes of NSF. This study suggests that NSF may be more prevalent than previously thought.
Is kidney dysfunction always present?
All the reported patients with NSF had underlying renal impairment. The renal dysfunction ranged from acute kidney injury to advanced chronic kidney disease (estimated GFR < 30 mL/minute/1.73 m2) and end-stage renal disease on renal replacement therapy, ie, hemodialysis or peritoneal dialysis. The incidence of NSF does not seem to be related to the cause of the underlying kidney disease.
What other diseases or comorbidities can be associated with NSF?
It is still unclear why not every patient with advanced renal failure develops NSF after exposure to gadolinium.
A variety of complex diseases and conditions have been reported to be associated with NSF, with no clear-cut evidence of causality or trigger. These include hypercoagulability states, thrombotic events, surgical procedures (especially those with reconstructive vascular components), calciphylaxis, kidney transplantation, hepatic disease (hepatorenal syndrome, liver transplantation, and hepatitis B and C), idiopathic pulmonary fibrosis, systemic lupus erythematosus, hypothyroidism, elevated serum ionized calcium or serum phosphate, hyperparathyroidism, and metabolic acidosis. A possible explanation is that most of these conditions are associated with an increased use of MRI or MRA testing (eg, in the workup for kidney or liver transplantation).
Many drugs have also been reported to be associated with NSF, including high-dose erythropoietin,20 sevelamer (Renagel),21 and, conversely, lack of angiotensin-converting enzyme inhibitor therapy,22 but none of these findings has been reproduced to date.
GADOLINIUM CHARACTERISTICS AND PHARMACOKINETICS
Gadolinium is a rare-earth lanthanide metallic element (atomic number 64) that is used in MRI and MRA because of its paramagnetic properties that enhance the quality of imaging. Its ionic form (Gd3+) is highly toxic if injected intravenously, so it is typically bound to a “chelate” to decrease its toxicity.23 The chelate stabilizes Gd3+ and thereby prevents its dissociation in vivo. These Gd-chelates can be classified (Table 2) according to their charge (ionic vs nonionic) and their structure (linear vs cyclic).
Most of the reported cases of NSF have been in patients who received gadodiamide, a nonionic, linear agent. Why gadodiamide has the highest rates of association with NSF is still unclear; perhaps it is simply the most widely used agent. Also, linear Gd compounds may be less stable and more likely to dissociate in vivo. The updated FDA Public Health Advisory in May 2007 warned against the use of all gadolinium-containing contrast agents for MRI, not just gadodiamide.
After intravenous injection, Gd-chelate equilibrates rapidly (within 2 hours) in the extracellular space. Very little of it enters into cells or binds to proteins. It is eliminated unchanged in the glomerular filtrate with no tubular secretion. In a study by Joffe et al,24 the elimination half-life of gadodiamide in patients with severely reduced renal function was considerably longer than in healthy volunteers (34.3 hours ± 22.9 vs 1.3 hours ± 0.25).
Since gadolinium compounds are not protein-bound and have a limited volume of distribution, they are typically removed by hemodialysis. Joffe et al found that an average of 65% of the gadodiamide was removed in a single hemodialysis session. However, they did not describe the specific features of the hemodialysis session, and it took four hemodialysis treatments to remove 99% of a single dose of gadolinium.24 A dialysis membrane with high permeability (large pores) seems to increase the clearance of the Gd-chelate during hemodialysis.25
Peritoneal dialysis may not remove gadolinium as effectively: Joffe et al24 reported that after 22 days of continuous ambulatory peritoneal dialysis, only 69% of the total amount of gadodiamide had been excreted, suggesting a very low peritoneal clearance.
SPECULATIVE PATHOGENESIS
Although a causal relationship between gadolinium use in patients with renal dysfunction and NSF has not been definitively established, the data derived from case reports assuredly raise this suspicion. Furthermore, on biopsy, gadolinium can be found in the skin of patients with NSF, adding evidence of causality.26–28
The mechanism by which Gd3+ might trigger NSF is still not understood. A plausible speculation is that if renal function is reduced, the half-life of the Gd-chelate molecule is significantly increased, as is the chance of Gd3+ dissociating from its chelate, leading to increased tissue exposure. Vascular trauma and endothelial dysfunction may allow free Gd3+ to enter tissues more easily, where macrophages phagocytose the metal, produce local profibrotic cytokines, and send out signals that recruit circulating fibrocytes to the tissues. Once in tissues, circulating fibrocytes induce a fibrosing process that is indistinguishable from normal scar formation.29
TREATMENTS LACK DATA
There is no consistently successful treatment for NSF.
In isolated reports, successful kidney transplantation slowed the skin fibrosis, but these findings need to be confirmed.30,31 Data from case reports should be interpreted very cautiously, as they are by nature sporadic and anecdotal. Moreover most of the reports of NSF were published on Web sites or as editorials and did not undergo exhaustive peer review. Because the evidence is weak, kidney transplantation should not be recommended as a treatment for NSF.
Oral steroids, plasmapheresis, extracorporeal photopheresis, thalidomide, topical ultraviolet-A therapy, and other treatments have yielded very conflicting results, with only anecdotal improvement of symptoms. In a recent case report,32 the use of intravenous sodium thiosulfate in addition to aggressive physical therapy provided some benefit by reducing the pain and improving the skin lesions.
Because of the lack of strong evidence of efficacy, we cannot advocate the use of any of these treatments until larger clinical trial results are available. Aggressive physical therapy along with appropriate pain control may have benefits and should be offered to all patients suffering from NSF.
Avoid gadolinium exposure in patients with renal insufficiency
The FDA33 recently asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents (Magnevist, MultiHance, Omniscan, Opti-MARK, ProHance), due to risk of NSF in patients with acute or chronic severe renal insufficiency (GFR < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
For the time being, gadolinium should be contraindicated in patients with acute kidney injury and chronic kidney disease stages 4 and 5 and in those who are on renal replacement therapy (either hemodialysis or peritoneal dialysis). If an MRI study with gadolinium-based contrast is absolutely required in a patient with end-stage renal disease or advanced chronic kidney disease, an agent other than gadodiamide should be used in the lowest possible dose.
Will hemodialysis prevent NSF?
In a patient who is already on hemodialysis, it seems prudent to perform hemodialysis soon after gadolinium exposure and again the day after exposure to increase gadolinium elimination. However, to date, there are no data to support the theory that doing this will prevent NSF.
Because peritoneal dialysis has been reported to clear gadolinium poorly, use of gadolinium is contraindicated. If gadolinium is absolutely needed, either more-aggressive peritoneal dialysis (keeping the abdomen “wet”) or temporary hemodialysis may be considered.
For patients with advanced chronic kidney disease who are not yet on renal replacement therapy, the use of gadolinium is contraindicated, and hemodialysis should not be empirically recommended after gadolinium exposure because we have no evidence to support its utility and because hemodialysis may cause harm.
Nephrology consultation should be considered before any gadolinium use in a patient with impaired renal function, whether acute or chronic.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18:614–617.
- Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003; 15:785–790.
- Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003; 139:903–906.
- Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol 2006; 54:S31–S34.
- Jimenez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum 2004; 50:2660–2666.
- Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 2006; 35:238–249.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Cowper SE, Bucala R, Leboit PE. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis—setting the record straight. Semin Arthritis Rheum 2006; 35:208–210.
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004; 36:598–606.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21:1104–1108.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17:2359–2362.
- Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007; 242:647–649.
- Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007; 188:586–592.
- Khurana A, Runge VM, Narayanan M, Greene JF, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol 2007; 42:139–145.
- Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007; 2:264–267.
- US Centers for Disease Control and Prevention (CDC). Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002–2006. MMWR Morb Mortal Wkly Rep 2007; 56:137–141.
- Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007; 243:148–157.
- Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 2007 May 4; e-pub ahead of print.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Jain SM, Wesson S, Hassanein A, et al. Nephrogenic fibrosing dermopathy in pediatric patients. Pediatr Nephrol 2004; 19:467–470.
- Fazeli A, Lio PA, Liu V. Nephrogenic fibrosing dermopathy: are ACE inhibitors the missing link? (Letter). Arch Dermatol 2004; 140:1401.
- Bellin MF. MR contrast agents, the old and the new. Eur J Radiol 2006; 60:314–323.
- Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 1998; 5:491–502.
- Ueda J, Furukawa T, Higashino K, et al. Permeability of iodinated and MR contrast media through two types of hemodialysis membrane. Eur J Radiol 1999; 31:76–80.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis, J Am Acad Dermatol 2007; 56:710–712.
- Perazella MA. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: is there a link? Clin J Am Soc Nephrol 2007; 2:200–202.
- Cowper SE. Nephrogenic systemic fibrosis: The nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis 2005; 46:763–765.
- Jan F, Segal JM, Dyer J, LeBoit P, Siegfried E, Frieden IJ. Nephrogenic fibrosing dermopathy: two pediatric cases. J Pediatr 2003; 143:678–681.
- Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure—role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol 2007; 2:258–263.
- US Food and Drug Administration. Accessed 01/03/08. http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18:614–617.
- Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003; 15:785–790.
- Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003; 139:903–906.
- Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol 2006; 54:S31–S34.
- Jimenez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum 2004; 50:2660–2666.
- Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 2006; 35:238–249.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Cowper SE, Bucala R, Leboit PE. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis—setting the record straight. Semin Arthritis Rheum 2006; 35:208–210.
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004; 36:598–606.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21:1104–1108.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17:2359–2362.
- Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007; 242:647–649.
- Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007; 188:586–592.
- Khurana A, Runge VM, Narayanan M, Greene JF, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol 2007; 42:139–145.
- Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007; 2:264–267.
- US Centers for Disease Control and Prevention (CDC). Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002–2006. MMWR Morb Mortal Wkly Rep 2007; 56:137–141.
- Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007; 243:148–157.
- Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 2007 May 4; e-pub ahead of print.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Jain SM, Wesson S, Hassanein A, et al. Nephrogenic fibrosing dermopathy in pediatric patients. Pediatr Nephrol 2004; 19:467–470.
- Fazeli A, Lio PA, Liu V. Nephrogenic fibrosing dermopathy: are ACE inhibitors the missing link? (Letter). Arch Dermatol 2004; 140:1401.
- Bellin MF. MR contrast agents, the old and the new. Eur J Radiol 2006; 60:314–323.
- Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 1998; 5:491–502.
- Ueda J, Furukawa T, Higashino K, et al. Permeability of iodinated and MR contrast media through two types of hemodialysis membrane. Eur J Radiol 1999; 31:76–80.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis, J Am Acad Dermatol 2007; 56:710–712.
- Perazella MA. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: is there a link? Clin J Am Soc Nephrol 2007; 2:200–202.
- Cowper SE. Nephrogenic systemic fibrosis: The nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis 2005; 46:763–765.
- Jan F, Segal JM, Dyer J, LeBoit P, Siegfried E, Frieden IJ. Nephrogenic fibrosing dermopathy: two pediatric cases. J Pediatr 2003; 143:678–681.
- Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure—role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol 2007; 2:258–263.
- US Food and Drug Administration. Accessed 01/03/08. http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm.
KEY POINTS
- NSF seems to arise in roughly 3% of patients with renal insufficiency who receive gadolinium, although the data are somewhat sketchy and the true incidence might be higher if the NSF is specifically looked for.
- Manufacturers of all available gadolinium contrast agents now must include a boxed warning about the risk of NSF in patients with acute or chronic severe renal insufficiency (glomerular filtration rate < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
- As yet, we have no effective treatment for NSF. If the patient is already on hemodialysis, it may be reasonable to perform hemodialysis immediately after exposure to gadolinium and again the next day.