User login
Spondylolysis and Spondylolisthesis Primary Care Clinicians' Role
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
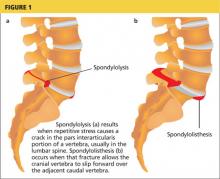
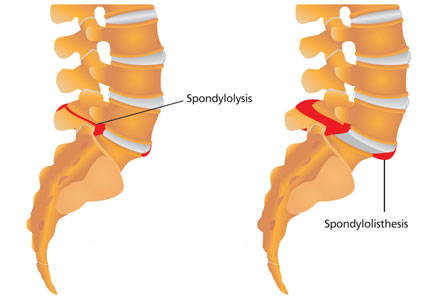
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
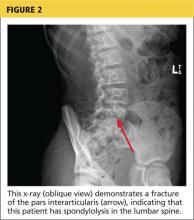
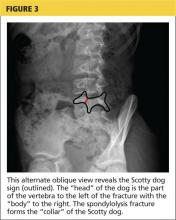
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
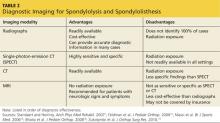
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
Girl, 13, With a Bump on Her Leg
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
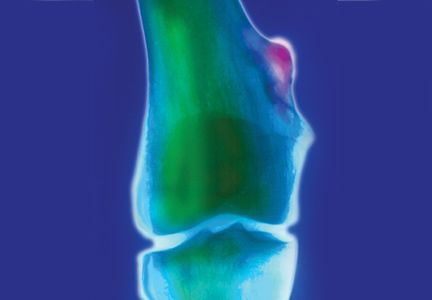
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
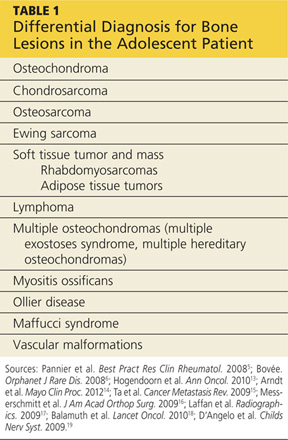
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).
Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).

Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).

Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.