User login
FDA Clears Imaging App for iPhone/iPad
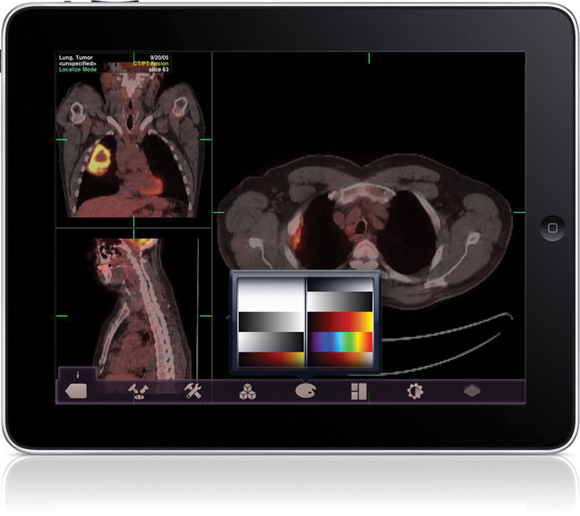
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
CDC Funds Research to Prevent Healthcare Acquired Infections
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
CDC Funds Research to Prevent Healthcare Acquired Infections
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
CDC Funds Research to Prevent Healthcare Acquired Infections
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
The Centers for Disease Control and Prevention is awarding a total of $10 million to five research groups in the latest round of funding for new research to help reduce healthcare setting–associated infections.
This will be the fourth round of "Prevention Epicenter" funding since the CDC began the program in 1997. The program is intended to discover better ways to deal with difficult health care problems such as antibiotic resistance and hospital infection control. According to CDC estimates, 1 in 20 hospitalized patients will acquire a healthcare-associated infection (HAI).
The CDC names new Prevention Epicenters every 5 years based on peer-reviewed grant applications.
This year’s awardees are: the Chicago Antimicrobial Resistance and Infection Prevention Epicenter (Cook County Health & Hospital System and Rush University Medical Center); the Duke University Prevention Epicenter in Durham, N.C.; the Translation Prevention Research Epicenter (Harvard Pilgrim Health Care) in Wellesley, Mass.; Southeastern Pennsylvania Adult and Pediatric Prevention Epicenter Network (University of Pennsylvania) in Philadelphia; and Washington University and BJC Epi-Center for Prevention of Healthcare Associated Infections in St. Louis.
Strategies the epicenters will be exploring include using combinations of bleach and ultraviolet light to clean hospital rooms, using new tests to distinguish patients who need antibiotics from those who don’t, finding ways to anticipate which medical devices are on the verge of causing an infection in a specific patient, and preventing HAIs by treating patients with probiotics.
In a press release announcing the awards, Dr. John Jernigan, director of CDC’s Office of HAI Prevention Research and Evaluation, said, "The Prevention Epicenter program discovers solutions and refines them so they can work to prevent infections for all healthcare settings. During the past decade, some of our biggest breakthroughs in health care infection prevention have been rooted in research of the Prevention Epicenter program, and we look forward to future advances."
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
FDA Updates Ambrisentan Label; Monthly Liver Enzyme Tests No Longer Required
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
FOOD AND DRUG ADMINISTRATION
FDA Updates Ambrisentan Label; Monthly Liver Enzyme Tests No Longer Required
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
The Food and Drug Administration on March 4 removed a warning pertaining to liver injury from the boxed warning on the ambrisentan label.
After a review of clinical trial data and postmarketing safety information, the FDA determined that ambrisentan (Letairis) presents only a small risk of liver injury and decided that monthly serum liver enzyme tests will no longer be required. The agency added that health care professionals should order liver enzyme tests if deemed clinically necessary. Ambrisentan is approved for the treatment of pulmonary arterial hypertension (PAH).
The boxed warning will continue to contain cautions about the use of ambrisentan during pregnancy. Preclinical studies showed that the drug can cause serious birth defects in animals. The drug will continue to be available only through a restricted distribution program called the Letairis Education and Access Program (LEAP).
In women of childbearing potential, the LEAP program requires evidence of a monthly pregnancy test before ambrisentan may be shipped.
Ambrisentan is an endothelin receptor antagonist. Endothelin is a naturally occurring substance that causes blood vessels to narrow, preventing normal blood flow in people with PAH. Ambrisentan has been shown to improve patients’ ability to exercise and to slow the progression of the disease.
FOOD AND DRUG ADMINISTRATION
Physician Recs Associated With Greater Infant Immunization
VANCOUVER, B.C. – Vaccine information provided by a child's doctor was the main driver of whether infants received their immunizations on time, a study of 254 women and their infants has shown.
Compared with women who received vaccine information from other sources (such as nurses, relatives, or the Internet), women who received their information from a physician were 2.98 times more likely to have their children fully immunized by the age of 3 months, according to Gina Calarco, who announced the results during a press briefing at the meeting.
“This is very significant in that doctors play a significant role in the education of the moms for vaccine purposes,” said Ms. Calarco, who is project manager at Infectious Diseases and Vaccines for Quintiles, an Overland Park, Kan., contract research organization.
Of the mothers in the study, 69% agreed to have a tetanus-diphtheria-pertussis (Tdap) booster vaccine themselves post partum, and 88% of their infants were fully vaccinated by their 2-month postpartum visit, according to immunization records. Of the 31% of mothers who declined the Tdap vaccine, 85% went on to have their infants vaccinated. That difference was not statistically significant. The investigators had hypothesized that the mothers who accepted Tdap vaccinations for themselves would be more likely to get their infants fully vaccinated, but this was not the case. Advice from the child's physician, however, was a significant factor.
Investigators sent questionnaires to the mothers after the 2-month well-child visit, receiving 105 responses. Of the mothers of fully vaccinated infants, 79% received vaccine information from a physician. Mothers of infants who were not fully vaccinated said they received vaccine information from a nurse (67%) or another source (79%).
“I think what this data overall shows is that physicians shouldn't be afraid to bring up [immunization], and should actively bring it up with their patients,” Ms. Calarco said. “Having the discussion – whether the parent asks for it or not – is the important part, and giving them that education on vaccination. I don't know that there's a right or wrong way [to do this], because everyone's different and everyone takes in information differently. But I think physicians need to be confident that they're heard and that the communication with their patient is effective.”
The study investigators acknowledged support from the Kenneth and Eva Smith Foundation and the University of Missouri–Kansas City.
VANCOUVER, B.C. – Vaccine information provided by a child's doctor was the main driver of whether infants received their immunizations on time, a study of 254 women and their infants has shown.
Compared with women who received vaccine information from other sources (such as nurses, relatives, or the Internet), women who received their information from a physician were 2.98 times more likely to have their children fully immunized by the age of 3 months, according to Gina Calarco, who announced the results during a press briefing at the meeting.
“This is very significant in that doctors play a significant role in the education of the moms for vaccine purposes,” said Ms. Calarco, who is project manager at Infectious Diseases and Vaccines for Quintiles, an Overland Park, Kan., contract research organization.
Of the mothers in the study, 69% agreed to have a tetanus-diphtheria-pertussis (Tdap) booster vaccine themselves post partum, and 88% of their infants were fully vaccinated by their 2-month postpartum visit, according to immunization records. Of the 31% of mothers who declined the Tdap vaccine, 85% went on to have their infants vaccinated. That difference was not statistically significant. The investigators had hypothesized that the mothers who accepted Tdap vaccinations for themselves would be more likely to get their infants fully vaccinated, but this was not the case. Advice from the child's physician, however, was a significant factor.
Investigators sent questionnaires to the mothers after the 2-month well-child visit, receiving 105 responses. Of the mothers of fully vaccinated infants, 79% received vaccine information from a physician. Mothers of infants who were not fully vaccinated said they received vaccine information from a nurse (67%) or another source (79%).
“I think what this data overall shows is that physicians shouldn't be afraid to bring up [immunization], and should actively bring it up with their patients,” Ms. Calarco said. “Having the discussion – whether the parent asks for it or not – is the important part, and giving them that education on vaccination. I don't know that there's a right or wrong way [to do this], because everyone's different and everyone takes in information differently. But I think physicians need to be confident that they're heard and that the communication with their patient is effective.”
The study investigators acknowledged support from the Kenneth and Eva Smith Foundation and the University of Missouri–Kansas City.
VANCOUVER, B.C. – Vaccine information provided by a child's doctor was the main driver of whether infants received their immunizations on time, a study of 254 women and their infants has shown.
Compared with women who received vaccine information from other sources (such as nurses, relatives, or the Internet), women who received their information from a physician were 2.98 times more likely to have their children fully immunized by the age of 3 months, according to Gina Calarco, who announced the results during a press briefing at the meeting.
“This is very significant in that doctors play a significant role in the education of the moms for vaccine purposes,” said Ms. Calarco, who is project manager at Infectious Diseases and Vaccines for Quintiles, an Overland Park, Kan., contract research organization.
Of the mothers in the study, 69% agreed to have a tetanus-diphtheria-pertussis (Tdap) booster vaccine themselves post partum, and 88% of their infants were fully vaccinated by their 2-month postpartum visit, according to immunization records. Of the 31% of mothers who declined the Tdap vaccine, 85% went on to have their infants vaccinated. That difference was not statistically significant. The investigators had hypothesized that the mothers who accepted Tdap vaccinations for themselves would be more likely to get their infants fully vaccinated, but this was not the case. Advice from the child's physician, however, was a significant factor.
Investigators sent questionnaires to the mothers after the 2-month well-child visit, receiving 105 responses. Of the mothers of fully vaccinated infants, 79% received vaccine information from a physician. Mothers of infants who were not fully vaccinated said they received vaccine information from a nurse (67%) or another source (79%).
“I think what this data overall shows is that physicians shouldn't be afraid to bring up [immunization], and should actively bring it up with their patients,” Ms. Calarco said. “Having the discussion – whether the parent asks for it or not – is the important part, and giving them that education on vaccination. I don't know that there's a right or wrong way [to do this], because everyone's different and everyone takes in information differently. But I think physicians need to be confident that they're heard and that the communication with their patient is effective.”
The study investigators acknowledged support from the Kenneth and Eva Smith Foundation and the University of Missouri–Kansas City.
Abortion Rate Fell in 2007
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
FROM MMWR
Abortion Rate Fell in 2007
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
The rate of legal abortion in the United States fell 2% in 2007, resuming a steady decline that began in 1998 but was interrupted by a small increase in 2006.
The new data were released by the Centers for Disease Control and Prevention on Feb. 24 in the Morbidity and Mortality Weekly Report (MMWR Feb. 25, 2011;60[ss01]:1-39).
Among 49 reporting areas, there were 827,609 legal induced abortions in the United States in 2007. Reporting is not mandatory, and California, Maryland, and New Hampshire do not report abortion data to the CDC. Only 45 states and territories reported abortion numbers in every year since 1998, when CDC began collecting the data. Among those 45 reporting areas, there were 810,582 abortions in 2007.
Within those areas the overall abortion rate in 2007 was 16.0 abortions/1,000 women aged 15-44 years, representing a 2% decline from 2006. The abortion ratio was 231 abortions/1,000 live births, a 3% decline from 2006.
From 1998 to 2007, the total number of abortions in the 45 consistently reporting areas declined 6%, the abortion rate declined 7%, and the abortion ratio declined 14%.
In 2007, 48 areas reported data on the age of women who underwent abortions. Within those areas, women aged 20-29 years accounted for 56.9% of all abortions. Adolescents (19 years of age and younger) accounted for 16.7% abortions; the rate of abortions among adolescents was 10.7/1,000, and the abortion ratio was 337 abortions/1,000 live births. Women aged 18-19 years accounted for 62.3% of the abortions among adolescents.
In 2007, 42 areas reported gestational age. Within those areas 62.3% of all abortions were obtained at 8 weeks’ gestation or less, and 91.5% were obtained at 13 weeks or less. Only 1.3% of abortions were obtained at 21 weeks’ gestation or longer.
Since 1998, women have been obtaining abortions earlier in gestation. Among the areas consistently reporting these data, there has been a 14% increase in the number of abortions obtained at 8 weeks’ of gestation or less.
Since the Food and Drug Administration approved mifepristone in September 2000, the proportion of medical abortions has increased 243%. In 2007, 13.1% of all abortions were performed by medical rather than surgical means.
FROM MMWR
Flu Vaccine Derived From Cell Culture Is Effective
A cell-culture–derived vaccine was 71% effective at protecting against seasonal influenza, according to results of a large randomized, controlled trial published online in the Lancet Feb. 16.
The study’s results "suggest that the Vero-cell–derived influenza vaccine is at least as effective in preventing culture-confirmed influenza infection as inactivated and live attenuated egg-derived vaccines," the study investigators said.
In addition, cell-culture vaccines might lead to more reliable vaccine supplies and better matches with prevalent influenza strains, they noted.
Only 23 of the 3,619 study participants (0.6%) who were given the vaccine during the 2008-2009 influenza season developed laboratory-confirmed influenza, compared with 80 of the 3,617 participants (2.2%) given placebo. The investigators calculated the vaccine’s protective efficacy to be 71.5% (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(10)62228-3]).
The protective efficacy was 78.5% against the three influenza strains contained in the vaccine, explained P. Noel Barrett, Ph.D., of Baxter BioScience (Orth/Donau, Austria), and colleagues.
Seasonal influenza vaccines are typically derived from embryonated hens’ eggs – a cumbersome practice with a host of manufacturing problems that often result in vaccine shortages. In contrast, vaccines derived from cell culture are made in standardized, closed manufacturing systems, allowing for a more reliable production process, the study’s authors noted.
The use of vaccines from cell culture might improve the effectiveness of seasonal influenza vaccines, the investigators said. With vaccines derived from hens’ eggs, investigators must make educated guesses on the influenza strains that will be prevalent more than 6 months into the future. With cell-culture–derived vaccines, however, predicted seasonal strains could be incorporated much closer to flu season.
Investigators recruited study participants at 36 centers in the United States between Dec. 1 and Dec. 15, 2008. The participants were healthy adults aged 18-49 years who received intramuscular injections either of a Vero-cell–derived, trivalent, inactivated-influenza vaccine or placebo. They returned to the clinics 18-24 days after vaccine for a blood draw to determine hemagglutination titres. At that time, participants were instructed to return once again within 48 hours of the onset of influenza-like symptoms. At those visits, investigators obtained nasopharyngeal swabs for culturing and typing viruses.
Adverse events associated with the vaccine tended to be mild or moderate. Among the vaccine recipients, 43% reported injection-site pain, compared with 8% of those receiving placebo. Other adverse events substantially more common in vaccine recipients were myalgia (18% vs. 6%), malaise (14% vs. 8%), fatigue, (18% vs. 12%), and headache (18% vs. 13%).
Compared with vaccines derived from embryonated eggs, vaccines derived from cell culture have several advantages, Dr. W. Paul Glezen of Baylor College of Medicine, Houston, wrote in an accompanying editorial (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(11)60174-8]).
"These advantages include availability and flexibility in use of tissue culture compared with the seasonality of use of hens’ eggs which, for the large quantities needed, must be scheduled in advance," Dr. Glezen explained. "Egg-based production processes are susceptible to microbial contamination, which has delayed vaccine production at some manufacturing sites in recent years."
In addition, some human influenza viruses such as subtype A H3N2 could be difficult to grow in eggs to generate the amount needed for vaccine production, he noted. And avian viruses such as subtype A H5N1 that are potential pandemic threats might prove lethal to chick embryos.
"The advantages of tissue-culture substrates allow a shorter production time between the annual determination of the vaccine’s formula and the distribution of vaccine," Dr. Glezen wrote. "The use of a tissue-culture substrate could shorten production time by 10 weeks, which might be crucial in a pandemic alert. Furthermore, production of vaccine antigens in tissue culture provides a safer vaccine for individuals who are allergic to eggs."
Dr. Glezen noted that the most important advantage of tissue-culture-grown influenza antigens might be the ability to preserve the structure of the hemagglutinin’s antibody-combining sites.
"Hemagglutinin is the most important surface antigen on the influenza virus, and protection is generally defined by the robustness of the antibody response to this antigen," he explained. However, adapting human influenza viruses to grow in the chick embryo alters the hemagglutinin antigen.
"Animal studies suggest that the use of vaccine virus antigens grown in mammalian cells could enhance protection after challenge with the human virus," Dr. Glezen said. "Also, the immune response might be broader than that produced by egg-grown antigens, which allows the possibility of better protection against new variants of prevalent viruses."
In addition, shortened production times would allow more time to make decisions about which influenza strains to include in influenza vaccines, he noted.
The DynPort Vaccine Company, with support from the U.S. government, funded the study. Two of the study authors hold patents on influenza vaccines derived from Vero-cell cultures. Six of the authors are employed by Baxter BioScience and have equity interests in the company. Two others are employed by DynPort, and one has an equity interest. Dr. Glezen disclosed serving as a coinvestigator for a grant funded by MedImmune Vaccines, and having received honoraria from AstraZeneca.
A cell-culture–derived vaccine was 71% effective at protecting against seasonal influenza, according to results of a large randomized, controlled trial published online in the Lancet Feb. 16.
The study’s results "suggest that the Vero-cell–derived influenza vaccine is at least as effective in preventing culture-confirmed influenza infection as inactivated and live attenuated egg-derived vaccines," the study investigators said.
In addition, cell-culture vaccines might lead to more reliable vaccine supplies and better matches with prevalent influenza strains, they noted.
Only 23 of the 3,619 study participants (0.6%) who were given the vaccine during the 2008-2009 influenza season developed laboratory-confirmed influenza, compared with 80 of the 3,617 participants (2.2%) given placebo. The investigators calculated the vaccine’s protective efficacy to be 71.5% (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(10)62228-3]).
The protective efficacy was 78.5% against the three influenza strains contained in the vaccine, explained P. Noel Barrett, Ph.D., of Baxter BioScience (Orth/Donau, Austria), and colleagues.
Seasonal influenza vaccines are typically derived from embryonated hens’ eggs – a cumbersome practice with a host of manufacturing problems that often result in vaccine shortages. In contrast, vaccines derived from cell culture are made in standardized, closed manufacturing systems, allowing for a more reliable production process, the study’s authors noted.
The use of vaccines from cell culture might improve the effectiveness of seasonal influenza vaccines, the investigators said. With vaccines derived from hens’ eggs, investigators must make educated guesses on the influenza strains that will be prevalent more than 6 months into the future. With cell-culture–derived vaccines, however, predicted seasonal strains could be incorporated much closer to flu season.
Investigators recruited study participants at 36 centers in the United States between Dec. 1 and Dec. 15, 2008. The participants were healthy adults aged 18-49 years who received intramuscular injections either of a Vero-cell–derived, trivalent, inactivated-influenza vaccine or placebo. They returned to the clinics 18-24 days after vaccine for a blood draw to determine hemagglutination titres. At that time, participants were instructed to return once again within 48 hours of the onset of influenza-like symptoms. At those visits, investigators obtained nasopharyngeal swabs for culturing and typing viruses.
Adverse events associated with the vaccine tended to be mild or moderate. Among the vaccine recipients, 43% reported injection-site pain, compared with 8% of those receiving placebo. Other adverse events substantially more common in vaccine recipients were myalgia (18% vs. 6%), malaise (14% vs. 8%), fatigue, (18% vs. 12%), and headache (18% vs. 13%).
Compared with vaccines derived from embryonated eggs, vaccines derived from cell culture have several advantages, Dr. W. Paul Glezen of Baylor College of Medicine, Houston, wrote in an accompanying editorial (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(11)60174-8]).
"These advantages include availability and flexibility in use of tissue culture compared with the seasonality of use of hens’ eggs which, for the large quantities needed, must be scheduled in advance," Dr. Glezen explained. "Egg-based production processes are susceptible to microbial contamination, which has delayed vaccine production at some manufacturing sites in recent years."
In addition, some human influenza viruses such as subtype A H3N2 could be difficult to grow in eggs to generate the amount needed for vaccine production, he noted. And avian viruses such as subtype A H5N1 that are potential pandemic threats might prove lethal to chick embryos.
"The advantages of tissue-culture substrates allow a shorter production time between the annual determination of the vaccine’s formula and the distribution of vaccine," Dr. Glezen wrote. "The use of a tissue-culture substrate could shorten production time by 10 weeks, which might be crucial in a pandemic alert. Furthermore, production of vaccine antigens in tissue culture provides a safer vaccine for individuals who are allergic to eggs."
Dr. Glezen noted that the most important advantage of tissue-culture-grown influenza antigens might be the ability to preserve the structure of the hemagglutinin’s antibody-combining sites.
"Hemagglutinin is the most important surface antigen on the influenza virus, and protection is generally defined by the robustness of the antibody response to this antigen," he explained. However, adapting human influenza viruses to grow in the chick embryo alters the hemagglutinin antigen.
"Animal studies suggest that the use of vaccine virus antigens grown in mammalian cells could enhance protection after challenge with the human virus," Dr. Glezen said. "Also, the immune response might be broader than that produced by egg-grown antigens, which allows the possibility of better protection against new variants of prevalent viruses."
In addition, shortened production times would allow more time to make decisions about which influenza strains to include in influenza vaccines, he noted.
The DynPort Vaccine Company, with support from the U.S. government, funded the study. Two of the study authors hold patents on influenza vaccines derived from Vero-cell cultures. Six of the authors are employed by Baxter BioScience and have equity interests in the company. Two others are employed by DynPort, and one has an equity interest. Dr. Glezen disclosed serving as a coinvestigator for a grant funded by MedImmune Vaccines, and having received honoraria from AstraZeneca.
A cell-culture–derived vaccine was 71% effective at protecting against seasonal influenza, according to results of a large randomized, controlled trial published online in the Lancet Feb. 16.
The study’s results "suggest that the Vero-cell–derived influenza vaccine is at least as effective in preventing culture-confirmed influenza infection as inactivated and live attenuated egg-derived vaccines," the study investigators said.
In addition, cell-culture vaccines might lead to more reliable vaccine supplies and better matches with prevalent influenza strains, they noted.
Only 23 of the 3,619 study participants (0.6%) who were given the vaccine during the 2008-2009 influenza season developed laboratory-confirmed influenza, compared with 80 of the 3,617 participants (2.2%) given placebo. The investigators calculated the vaccine’s protective efficacy to be 71.5% (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(10)62228-3]).
The protective efficacy was 78.5% against the three influenza strains contained in the vaccine, explained P. Noel Barrett, Ph.D., of Baxter BioScience (Orth/Donau, Austria), and colleagues.
Seasonal influenza vaccines are typically derived from embryonated hens’ eggs – a cumbersome practice with a host of manufacturing problems that often result in vaccine shortages. In contrast, vaccines derived from cell culture are made in standardized, closed manufacturing systems, allowing for a more reliable production process, the study’s authors noted.
The use of vaccines from cell culture might improve the effectiveness of seasonal influenza vaccines, the investigators said. With vaccines derived from hens’ eggs, investigators must make educated guesses on the influenza strains that will be prevalent more than 6 months into the future. With cell-culture–derived vaccines, however, predicted seasonal strains could be incorporated much closer to flu season.
Investigators recruited study participants at 36 centers in the United States between Dec. 1 and Dec. 15, 2008. The participants were healthy adults aged 18-49 years who received intramuscular injections either of a Vero-cell–derived, trivalent, inactivated-influenza vaccine or placebo. They returned to the clinics 18-24 days after vaccine for a blood draw to determine hemagglutination titres. At that time, participants were instructed to return once again within 48 hours of the onset of influenza-like symptoms. At those visits, investigators obtained nasopharyngeal swabs for culturing and typing viruses.
Adverse events associated with the vaccine tended to be mild or moderate. Among the vaccine recipients, 43% reported injection-site pain, compared with 8% of those receiving placebo. Other adverse events substantially more common in vaccine recipients were myalgia (18% vs. 6%), malaise (14% vs. 8%), fatigue, (18% vs. 12%), and headache (18% vs. 13%).
Compared with vaccines derived from embryonated eggs, vaccines derived from cell culture have several advantages, Dr. W. Paul Glezen of Baylor College of Medicine, Houston, wrote in an accompanying editorial (Lancet; 2011 Feb. 16 [doi:10.1016/S0140-6736(11)60174-8]).
"These advantages include availability and flexibility in use of tissue culture compared with the seasonality of use of hens’ eggs which, for the large quantities needed, must be scheduled in advance," Dr. Glezen explained. "Egg-based production processes are susceptible to microbial contamination, which has delayed vaccine production at some manufacturing sites in recent years."
In addition, some human influenza viruses such as subtype A H3N2 could be difficult to grow in eggs to generate the amount needed for vaccine production, he noted. And avian viruses such as subtype A H5N1 that are potential pandemic threats might prove lethal to chick embryos.
"The advantages of tissue-culture substrates allow a shorter production time between the annual determination of the vaccine’s formula and the distribution of vaccine," Dr. Glezen wrote. "The use of a tissue-culture substrate could shorten production time by 10 weeks, which might be crucial in a pandemic alert. Furthermore, production of vaccine antigens in tissue culture provides a safer vaccine for individuals who are allergic to eggs."
Dr. Glezen noted that the most important advantage of tissue-culture-grown influenza antigens might be the ability to preserve the structure of the hemagglutinin’s antibody-combining sites.
"Hemagglutinin is the most important surface antigen on the influenza virus, and protection is generally defined by the robustness of the antibody response to this antigen," he explained. However, adapting human influenza viruses to grow in the chick embryo alters the hemagglutinin antigen.
"Animal studies suggest that the use of vaccine virus antigens grown in mammalian cells could enhance protection after challenge with the human virus," Dr. Glezen said. "Also, the immune response might be broader than that produced by egg-grown antigens, which allows the possibility of better protection against new variants of prevalent viruses."
In addition, shortened production times would allow more time to make decisions about which influenza strains to include in influenza vaccines, he noted.
The DynPort Vaccine Company, with support from the U.S. government, funded the study. Two of the study authors hold patents on influenza vaccines derived from Vero-cell cultures. Six of the authors are employed by Baxter BioScience and have equity interests in the company. Two others are employed by DynPort, and one has an equity interest. Dr. Glezen disclosed serving as a coinvestigator for a grant funded by MedImmune Vaccines, and having received honoraria from AstraZeneca.
FROM THE LANCET
Major Finding: A seasonal influenza vaccine derived from a Vero-cell culture was 71.5% effective in preventing influenza.
Data Source: Randomized, double-blind, placebo-controlled study of 7,250 adults aged 18-49 years, during the 2008-2009 season.
Disclosures: The DynPort Vaccine Company, with support from the U.S. government, funded the study. Two of the study authors hold patents on influenza vaccines derived from Vero-cell cultures. Six of the authors are employed by Baxter BioScience and have equity interests in the company. Two others are employed by DynPort, and one has an equity interest.