User login
VTE PX in ED Admissions
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
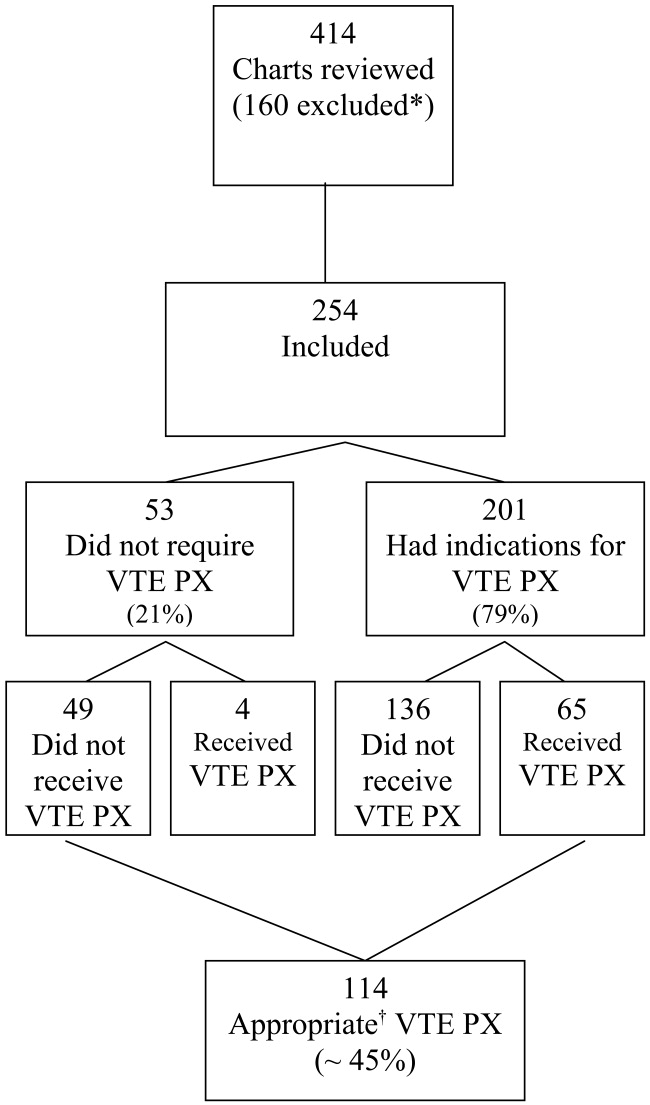
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).
Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
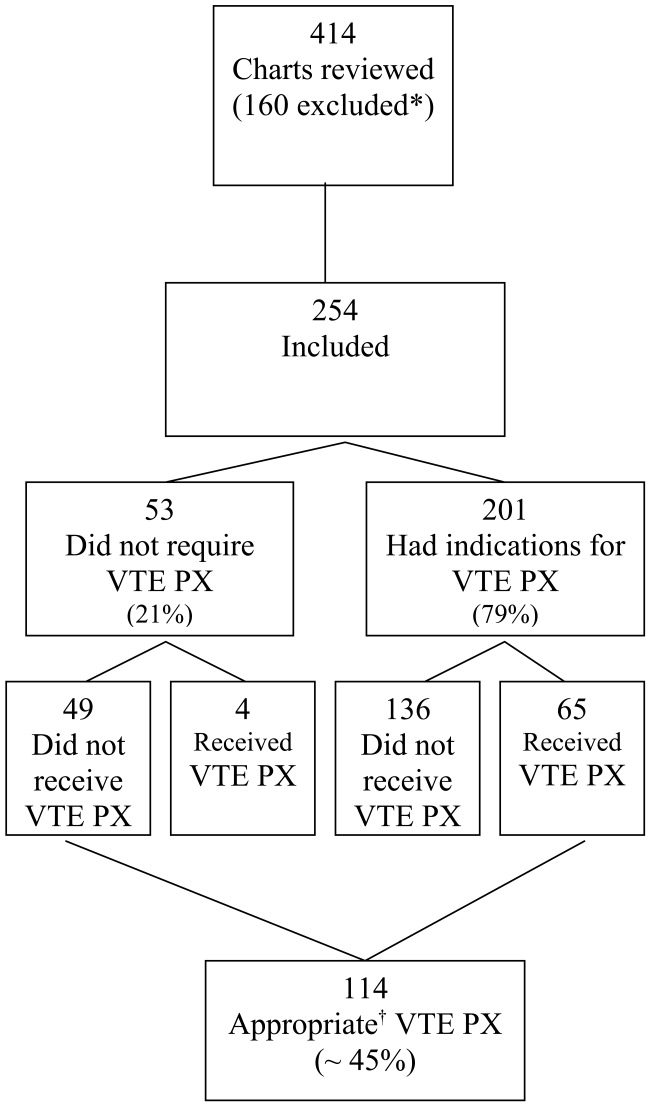
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).

Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).

Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
Copyright © 2007 Society of Hospital Medicine