User login
Decreasing the burden of postacute sequelae of SARS-CoV-2 infection: What we know
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.
In reply: Rapidly progressive pleural effusion
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
Rapidly progressive pleural effusion
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
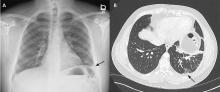
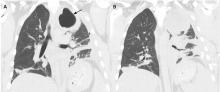
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
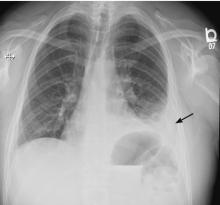
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
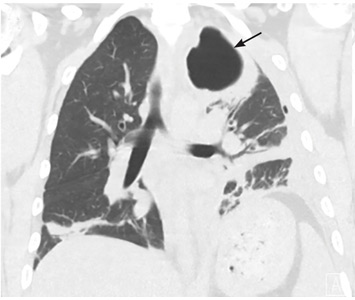
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
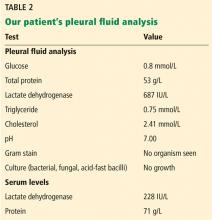
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
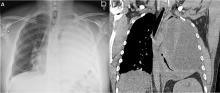
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503