User login
Age‐Specific CSF Protein Reference Values
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
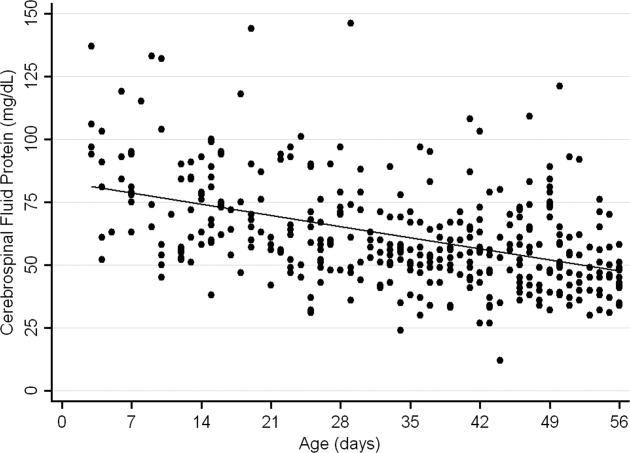
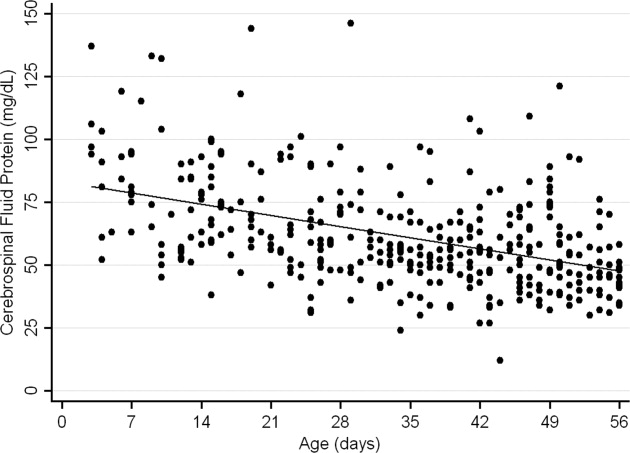
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0
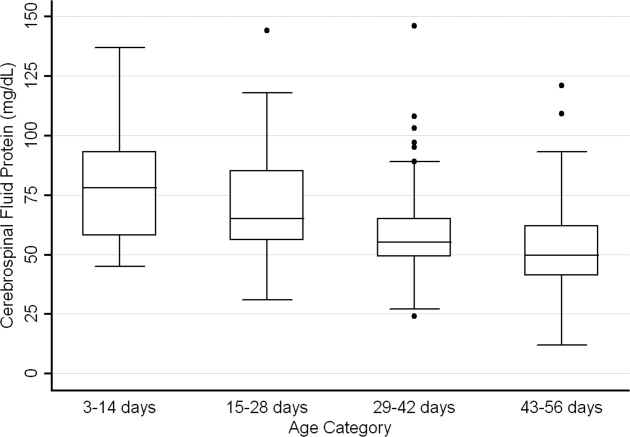
CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Copyright © 2010 Society of Hospital Medicine
Congenital Anomalies in Infant HSV
Herpes simplex virus (HSV) is a significant cause of pediatric hospitalization, morbidity and mortality, particularly in infants under 60 days of age, where HSV can present as meningoencephalitis, skin disease, or sepsis.14 Most prior studies use data from registries taken from single centers or a restricted group of hospitals. Thus, there is a paucity of recent, nationally‐representative information about the outcome of infants infected with HSV, especially those treated at nonteaching hospitals or with rarer comorbid conditions. The goal of this project was to determine the patient and hospital characteristics associated with worse clinical outcomes in infants under the age of 60 days admitted with HSV disease. We hypothesized that younger infants, infants with a concurrent congenital anomaly, and infants treated at non‐children's hospitals would have worse clinical outcomes. To answer these questions, we used 2003 panel data from the Healthcare Cost and Utilization Project (HCUP) Kids' Inpatient Database (KID), a nationally representative sample of inpatient hospitalizations in the United States.
Methods
Study Population and Data Collection
We conducted a retrospective population cohort study of all infants admitted at 60 days of age who were discharged with a diagnosis of HSV disease between January 1, 2003 and December 31, 2003, using the 2003 KID. The KID is a collaborative project between the Agency for Healthcare Research and Quality AHRQ and 36 states, which includes approximately 2.9 million pediatric discharge records from 3438 hospitals.5 The KID is the only national, all‐payer database of pediatric hospitalizations in the United States.
Patient Eligibility
As in prior studies,611 children were eligible for this project if they were discharged with an International Classification of Disease, ninth edition, Clinical Modification (ICD‐9CM) discharge code of 054.xx (herpes simplex virus), where xx represented any combination of one or two‐digit codes, or 771.2 (neonatal viral infection including HSV). However, the 771.2 code may also contain other perinatal infections of relatively rare frequency, such as toxoplasmosis. Thus, we also performed the same set of analyses on the cohort of children who had an 054.xx code alone. No results presented in this study changed in statistical significance when this smaller cohort of infants was examined.
Data Variables and Outcomes
Outcome Variables
We examined 2 primary clinical outcomes in this study: in‐hospital death and the occurrence of a serious complication. Complications were identified using ICD‐9CM codes from both prior work12 and examination of all diagnosis and procedure codes for eligible infants by the 2 principal investigators (Appendix). These 2 reviewers had to independently agree on the inclusion of an ICD‐9CM code as a complication. In‐hospital deaths were captured through a disposition code of 20 in the KID dataset. Length of stay (LOS) and in‐hospital costs were examined as secondary outcome measures for specific risk factors of interest.
Demographic and Comorbidity Variables
Demographic and comorbidity variables were included in the analyses to control for the increased cost, LOS, or risk of a complication that result from these factors.1315 Demographic information available in the KID included gender, age at admission, race, low birth weight infants, and insurance status. Age at admission was grouped into 4 categories: 07 days, 814 days, 1528 days, and 2960 days. Infants were classified as low birth weight if they had an ICD‐9CM code for a birth weight <2000 g (ICD‐9CM codes 765.01‐07, 765.11‐17, or 765.21‐27). We used the ICD‐9CM codes shown in the Appendix to classify various comorbid conditions. Because of the young age of the cohort, all comorbid conditions consisted of congenital anomalies that were grouped according to the involved organ system. To help classify patients by their illness severity, we used the All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) severity of illness classification for each hospital admission (3M Corporation, St. Paul, MN). The APR‐DRG classification system used discharge diagnoses, procedures, and demographic information to assign patients to 4 severity of illness categories.
Hospital Characteristics
We identified the following hospital characteristics from the KID: total bed size, divided as small, medium, and large; hospital status (children's hospital vs. non‐children's hospital, teaching hospital vs. nonteaching hospital); source of admission (emergency department, clinic, other hospitals); and location (rural vs. urban). Children's hospitals were identified by the AHRQ using information from the National Association of Children's Hospitals and Related Institutions, while teaching hospital status was determined by the presence of an approved residency program and a ratio of full‐time residents to beds of 0.25 or greater.5
Statistical Analysis
All analyses accounted for the complex sampling design with the survey commands included in STATA 9.2 (Statacorp, College Station, TX) and report national estimates from the data available in the 36 surveyed states. Because of the complex sampling design, the Wald test was used to determine significant differences for each outcome in univariable analysis. Variance estimates were reported as standard errors of the mean. We constructed multivariable logistic regression models to assess the adjusted impact of patient and hospital‐level characteristics on each primary outcome measure; ie, in‐hospital death and development of a serious complication. Negative binomial models were used for our secondary outcomes, LOS and costs, because of their rightward skew. Variance estimates for each model accounted for the clustering of data at the hospital level, and data were analyzed as per the latest AHRQ statistical update.16
Results
The 2003 KID identified 1587 hospitalizations for HSV in infants admitted at an age of 60 days or less in the entire United States. These infants had a total hospital cost of $27,147,000. Of the cohort, 10% had a concurrent congenital anomaly. Most infants (73.5%) were admitted within 14 days of birth, and 15.5% were transferred from another hospital. Based on APR‐DRG criteria, 33% of the infants were classified as having a moderate risk of death, 24% as major risk, and 12.2% as extreme risk. The majority of infants were treated at non‐children's hospitals (85.3%) in urban locations (91.5%). The average LOS was 12.0 0.6 days and the average total hospital cost was $17,382 1269. After admission, 267 of the infants, or 16.8%, had at least 1 serious complication. Fifty infants died during the hospitalization included in the KID.
Risk Factor Analysis
Serious Complications
Univariable (Table 1) analysis identified several factors associated with higher rates of serious complications. Younger age at admission was associated with a higher risk of serious complications. This trend was greatest for infants admitted under 14 days of age, of which 20.2% had a serious complication, compared with 10.2% of the infants admitted between 29 and 60 days of age. Infants with any identified congenital anomaly had significantly higher rates of serious complication (41.1% vs. 14.8% for infants without a congenital anomaly). Similar findings were seen with low birth weight infants. Infants who were transferred prior to the hospitalization captured in the KID had a higher complication rate (38.7%) than infants admitted as a routine admission (15.9%) or via the emergency room (8.8%). Among hospital‐level factors, infants admitted to children's or teaching hospitals had higher rates of serious complications, although only the difference between teaching and nonteaching hospitals reached statistical significance (Table 1).
| Patient‐Level Factors | % of Cohort | % with Serious Complication | % Death |
|---|---|---|---|
| |||
| Age at presentation | |||
| 7 days | 58.4 | 21.6* | 4.2* |
| 814 days | 15.1 | 15.8 | 3.6 |
| 1528 days | 16.4 | 9.7 | 2.1 |
| 2960 days | 10.1 | 10.2 | 0 |
| Low birth weight | |||
| Yes | 10.6 | 44.2* | 9.0* |
| No | 89.4 | 14.3 | 2.7 |
| Type of insurance | |||
| Private | 47.4 | 15.6 | 2.1* |
| Medicaid | 49.0 | 19.2 | 4.8 |
| Self pay | 3.6 | 17.0 | 0 |
| Race | |||
| White | 52.8 | 17.7 | 3.5 |
| Black | 18.9 | 17.6 | 4.2 |
| Other | 28.3 | 19.2 | 4.5 |
| Gender | |||
| Female | 45.4 | 15.7 | 2.2 |
| Male | 54.6 | 18.9 | 4.3 |
| Any congenital anomaly | |||
| Yes | 10.0 | 41.1* | 10.4* |
| No | 90.0 | 14.8 | 2.6 |
| Admission type | |||
| Routine | 62.3 | 15.9* | 2.8* |
| Emergency room | 22.2 | 8.8 | 1.1 |
| Transfer from another hospital | 15.5 | 38.7 | 9.6 |
| APR‐DRG risk | |||
| Mild | 3.0 | 0.3* | 0* |
| Moderate | 33.0 | 2.0 | 0.5 |
| Major | 24.0 | 24.7 | 2.3 |
| Extreme | 12.2 | 85.0 | 20.8 |
| Hospital‐level factors | |||
| Children's hospital | |||
| Yes | 14.7 | 27.0 | 6.4 |
| No | 85.3 | 16.3 | 3.1 |
| Teaching hospital | |||
| Yes | 68.4 | 21.3* | 4.3* |
| No | 31.7 | 8.5 | 1.5 |
| Location | |||
| Urban | 91.5 | 18.0* | 3.6 |
| Rural | 8.5 | 9.0 | 1.6 |
| Hospital size | |||
| Small | 14.1 | 19.3 | 4.2 |
| Medium | 25.9 | 14.3 | 3.2 |
| Large | 60.0 | 18.1 | 3.3 |
Many of these factors were independently associated with increased complication rates in multivariable analysis (Table 2). Infants under 7 days of age on admission (odds ratio [OR], 2.68; 95% confidence interval [CI], 1.112.47), low birth weight (OR, 5.17; 95% CI, 2.988.98), and the concurrent presence of a congenital anomaly (OR, 3.09; 95% CI, 1.805.33) were associated with higher odds of a serious complication. Site of care lost its statistical significance once our models adjusted for differences in illness severity. Insurance status, gender, and race were not associated with a change in complication rates for these infants.
| Risk Factor | Serious Complication | Mortality | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| ||||
| Age at admission | ||||
| 7 days | 2.68 | 1.112.47 | 1.63 | 0.347.73 |
| 814 days | 1.22 | 0.403.73 | 2.15 | 0.3612.9 |
| 1428 days | 0.87 | 0.322.37 | Reference* | |
| 2960 days | Reference | |||
| Racial/ethnic status | ||||
| White | Reference | Reference | ||
| Black | 0.90 | 0.451.82 | 1.30 | 0.433.89 |
| Other | 0.99 | 0.571.70 | 1.19 | 0.482.99 |
| Treatment at children's hospital | 2.33 | 0.836.18 | 2.59 | 0.6510.2 |
| Treatment at teaching hospital | 1.71 | 0.943.12 | 1.86 | 0.566.25 |
| Female gender | 0.96 | 0.631.48 | 0.28 | 0.100.82 |
| Medicaid insurance | 1.51 | 0.912.50 | 1.69 | 0.634.53 |
| Transferred from another hospital | 3.76 | 2.036.98 | 3.47 | 1.428.46 |
| Transferred to another hospital | 1.35 | 0.672.73 | ||
| Presence of a congenital anomaly | 3.09 | 1.805.33 | 4.26 | 1.7610.3 |
| Low birth weight infant | 5.17 | 2.988.98 | 5.33 | 1.9015.0 |
Death
Risk factors for higher mortality rates followed similar trends as those for the risk of a serious complication. Younger age at admission, low birth weight status, the presence of a serious complication, admission from another hospital, and treatment at a children's hospital or teaching hospital were all associated with higher mortality rates. In multivariable analysis, the concurrent presence of a congenital anomaly was associated with higher odds of death (OR, 4.26; 95% CI, 1.7610.3). The cause of increased death in infants with congenital anomalies appeared to be a higher rate of serious complications, as including serious complications in the multivariable regression model resulted in the association between congenital anomalies and death losing statistical significance (OR in revised model 1.95; 95% CI, 0.636.05). Site of care again was not associated with differences in mortality after controlling for patient case‐mix.
Concurrent Congenital Anomalies
Based on the higher complication and mortality rates seen in infants with HSV who had a concurrent congenital anomaly, we then investigated how the presence of specific congenital anomalies influenced clinical outcomes, LOS, and total hospital costs with HSV disease. Using the congenital anomaly groups listed in the Appendix, we found that congenital heart disease, central nervous system anomalies, pulmonary anomalies, and gastrointestinal anomalies were each associated with either higher rates of serious complications, longer LOS, or higher total hospital costs compared to infants without congenital anomalies (Table 3). Serious complications occurred most commonly in patients with central nervous system anomalies (55.6%) and congenital heart disease (50.8%), while infants with pulmonary anomalies had the longest LOS (37.1 10.0 days) and highest total hospital costs of all anomaly categories. The types of complications differed by the anomaly group: infants with cardiac and pulmonary anomalies had the highest rates of respiratory complications (45% and 40%, respectively), whereas those with central nervous system anomalies had the highest rates of cardiac complications (51%). Each anomaly class had a similar rate of neurological complications, between 30% and 40%.
| Number* | % With Serious Complication | LOS (days) | Total Hospital Costs (2003 dollars) | |
|---|---|---|---|---|
| ||||
| No congenital anomaly | 1391 | 14.8 | 11.3 0.6 | 15,118 1158 |
| Type of congenital anomaly | ||||
| Congenital heart disease | 73 | 50.8 | 23.5 4.6 | 46,760 9340 |
| Central nervous system anomaly | 31 | 55.6 | 15.4 3.0 | 23,962 5037 |
| Head/neck anomaly | 13 | 40.6 | 11.1 4.6 | 14,132 7860 |
| Pulmonary anomaly | 13 | 34.1 | 37.1 10.0 | 67,234 21,002 |
| Gastrointestinal anomaly | 20 | 33.5 | 21.6 4.9 | 41,207 13,878 |
| Genitourinary anomaly | 19 | 24.1 | 11.0 2.5 | 10,906 1890 |
| Musculoskeletal anomaly | ||||
| Genetic anomaly | 18 | 10.2 | 12.2 2.4 | 15,990 3808 |
Site of Care
Finally, we examined the LOS and costs of receiving care at a children's hospital. The data shown in Tables 1 and 2 suggest that receiving treatment at a children's hospital does not result in improved clinical outcomes for infants admitted with HSV. One potential advantage, though, is improved efficiency of care, which would result in a shorter LOS or lower costs. Using negative binomial multivariable regression models to account for differences in patient characteristics, regional variation, and insurance status, treatment at a children's hospital was associated with an 18% shorter LOS (95% CI, 1%34%) compared to non‐children's hospitals after accounting for the generally sicker infants treated at children's hospitals. Children's hospitals, though, were more expensive than non‐children's hospitals (increase of $642 per day; 95% CI, $2321052). These results remained consistent when we omitted transferred patients from the model, instead of controlling for them in the analysis.
Conclusions
There has been little prior information to guide practitioners and parents about factors that potentially influence clinical outcome of infants hospitalized with HSV in non‐children's hospitals, although over 80% of infants are managed at non‐children's hospitals. These studies also did not have the power to characterize the risk of poor clinical outcome associated with rarer clinical factors.1, 2, 6 This study, using nationally representative data, found that these rarer clinical factors and site of care may influence the outcomes of infants hospitalized with HSV, albeit in different methods. Younger age at admission and a coexisting congenital anomaly remained statistically significant predictors of worse clinical outcomes after controlling for various patient and hospital factors. Not all congenital anomalies increased the risk of death or serious complications; rather, anomalies that affected either the cardiopulmonary system or the central nervous system appeared to result in the highest increases in risk. This study also found that treatment of infants with HSV at a children's hospital was associated with a 28% shorter LOS after accounting for the sicker patients cared for by children's hospitals. This finding is in contrast to prior studies of common pediatric conditions, where there were no differences in the LOS between children's and non‐children's hospitals,17, 18 and severe sepsis, where children's hospitals had longer LOSs.19 These results confirm the importance of specific risk factors in predicting the likelihood that an infant admitted with HSV may have a poor clinical outcome. Also, these results emphasize the differences in outcomes that may occur at different types of hospitals.
This study is the first to find that certain congenital anomalies or conditions may be associated with worse clinical outcomes from HSV. There is little information in the literature to explain these findings. Those anomalies that affect the cardiopulmonary or central nervous system may either worsen the symptoms of HSV or predispose infants to have a serious complication, such as shock or respiratory failure. This finding would be similar to the increased risk of serious complications seen in infants with congenital heart disease who contract respiratory syncytial virus20 or infants with genetic syndromes who undergo heart surgery.21 Alternatively, because we do not have information on do‐not‐resuscitate status, the presence of one of these congenital anomalies may result in more withdrawal of care when an infant is infected with HSV and has a serious complication; the LOS of these children may not reflect these decisions because the decision to withdrawal care may only occur after the child's condition worsens significantly, which may happen any time during the disease course. However, this theory is less likely because we failed to find similar results with other congenital anomalies such as genetic or chromosomal syndromes. Further examination of these infants and their overall response to insults such as HSV is needed to understand how these anomalies influence the outcomes of a serious, unrelated illness.
Age upon admission was another important predictor of poor outcomes when analyzed in univariable or multivariable analysis. This result is consistent with prior work,14 which suggests that younger children are more likely to be hospitalized with either congenitally acquired HSV or systemic disease. The information contained in the KID does not allow us to determine whether young age is a risk factor for poor outcome irrespective of the clinical presentation of HSV, or whether age serves as a proxy for the appearance of more severe clinical disease. This effect of age remained present even after controlling for the higher risk of a serious complication and death in low birth weight infants. There are limited data that suggest that premature birth is an independent risk factor for worse outcomes associated with perinatal or congenital infection; 1 previous case study of Enterobacter sakazakii infections found a higher fatality rate for premature infants compared to term infants.22 This study supports these findings.
This study found that treatment at a children's hospital resulted in a 28% shorter LOS without a statistically significant difference in clinical outcomes after controlling for case‐mix differences. This finding is in contrast to prior studies of common pediatric conditions17, 18 and severe sepsis.19 There are several potential explanations for the difference in findings. For common pediatric conditions, there may be fewer variations in treatment style and less need for new diagnostic modalities that are more available at academic centers. For HSV disease, though, children's hospitals may also be more likely than non‐children's hospitals to perform polymerase‐chain reaction (PCR) testing for the diagnosis of perinatally acquired HSV, correctly identify the disorder, or receive the test results in a timely fashion. Pediatric subspecialists, such as infectious disease physicians or neurologists, are also likely to be more available at children's hospitals than at other centers. While the role of subspecialty consultation in improving outcomes for neonates with HSV is not known, improved outcomes at children's hospitals has been described for other serious conditions such as splenic injuries.23 Children's hospitals had higher daily costs than non‐children's hospitals, as has been found in other work.17, 19 Children's hospitals may be treating sicker patients, for whom we are unable to adequately adjust for their illness severity with hospital administrative data.17, 19 Also, there may be a greater use of medical tests and treatments that increase the costs of care. These costs do not include indirect costs to the families such as loss of work and travel costs. In light of the shorter LOS in children's hospitals, policy makers will need to balance the potentially higher daily costs of care with more efficient management of the disease process.
Because this study used hospital administrative records, there are a few limitations. We used ICD‐9CM diagnosis codes to identify patients, congenital anomalies, and complications. The diagnosis of some infants with HSV or less significant congenital anomalies could have been missed because clinicians either overlooked the disease or did not make the diagnosis before discharge. This form of spectrum bias would likely miss the infants with the least severe disease and make it more difficult to find the results that we found in this study.24 Prior work successfully used and validated similar ICD‐9CM codes to identify HSV cases among the different types of hospitals included in the KID.611 Our study design estimated 1587 cases of neonatal HSV in 2003. A prospective study of maternal serologic and virologic status during pregnancy estimated 480 to 2160 new cases of neonatal HSV per year.25 Thus, while miscoding is a potential limitation to our study, the overall numbers of patients in this study were similar to past annual estimates. One potential area of miscounting, though, was the inability of the KID to link the records of 16% of the identified infants with HSV whose care was transferred between hospitals. These infants may result in misleading LOS or cost information: lower for the transferring hospital, because they only kept the child a short period of time, or lower for the accepting hospital, as some of the total hospital stay is not accounted for in the KID. We accounted for this issue in 2 ways. First, we included a variable for being transferred in the multivariable models, and found no difference in any results when we omitted these patients from the analysis. Second, we performed a univariable analysis stratified by transfer status, which did not differ substantially from our main model for most variables. Accurate linkage of all the hospital records for an infant's hospital course, likely only through a mandatory reporting system for infant HSV, would help confirm the associations we identified in this study.
In conclusion, infants with congenital anomalies should be closely monitored for the development of serious complications associated with HSV, particularly those infants with congenital heart disease, pulmonary anomalies, or central nervous system anomalies. Closer investigation of the care practices that children's hospitals use in the management of infants with HSV is needed to improve the efficiency of care delivered to these infants, as HSV disease remains a significant public health problem.
- ,,, et al.Natural history of neonatal herpes simplex virus infections in the acyclovir era.Pediatrics.2001;108:223–229.
- ,,.Herpes simplex viruses.Clin Infect Dis.1998;26:541–553.
- ,,.Herpes simplex virus infections. In: Remington JS, Wilson CB, Baker CJ, editors.Infectious Diseases of the Fetus and Newborn Infant.5th ed.Philadelphia, PA:W.B. Saunders;2001. p425–446.
- ,,, et al.Changing presentation of herpes simplex virus infection in neonates.J Infect Dis.1988;158:109–116.
- Design of the HCUP Kids' Inpatient Database (KID), 2003. Healthcare Cost and Utilization Project (HCUP).Rockville, MD:Agency for Healthcare Research and Quality;2003. Revised January 30, 2006. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/KID_2003_Design_Edited_013006.pdf. Accessed October 2009.
- ,,.Incidence of neonatal herpes simplex virus infections in a managed‐care population.Sex Transm Dis.2007;34:704–708.
- ,,, et al.Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate.Am J Obstet Gynecol.2006;194:408–414.
- ,,, et al.The estimated economic burden of genital herpes in the united states.BMC Infect Dis.2001;1:5.
- ,,, et al.Accuracy of obstetric diagnoses and procedures in hospital discharge data.Am J Obstet Gynecol.2006;194:992–1001.
- ,,, et al.The epidemiology of neonatal herpes simplex virus infections in California from 1985 to 1995.J Infect Dis.1999;180:199–202.
- ,,.Medical care expenditures for genital herpes in the United States.Sex Transm Dis.2000;27:32–38.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,, et al.The importance of comorbidities in explaining differences in patient costs.Med Care.1996;34:767–782.
- ,,, et al.Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population‐based study.Arch Pediatr Adolesc Med.1997;151:1096–1103.
- ,,.The influence of chronic disease on resource utilization in common acute pediatric conditions. Financial concerns for children's hospitals.Arch Pediatr Adolesc Med.1999;153:169–179.
- Health Care Cost and Utility Project.Calculating Kids' Inpatient Database (KID) Variances. December 16, 2005. Methods Series Report # 2005‐5.Rockville, MD:Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/CalculatingKIDVariances.pdf. Accessed October2009.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Length of stay for common pediatric conditions: teaching versus nonteaching hospitals.Pediatrics.2003;112:278–281.
- ,,.Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- .Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection.J Pediatr.2003;143:S112–S117.
- ,,, et al.Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery.J Thorac Cardiovasc Surg.2007;133:1344–1353,1353,e1341–e1343.
- .Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature.Medicine.2001;80:113–122.
- ,,, et al.Hospital characteristics associated with the management of pediatric splenic injuries.JAMA.2005;294:2611–2617.
- ,.Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation.Ann Intern Med.2002;137:598–602.
- ,,, et al.Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant.JAMA.2003;289:203–209.
Herpes simplex virus (HSV) is a significant cause of pediatric hospitalization, morbidity and mortality, particularly in infants under 60 days of age, where HSV can present as meningoencephalitis, skin disease, or sepsis.14 Most prior studies use data from registries taken from single centers or a restricted group of hospitals. Thus, there is a paucity of recent, nationally‐representative information about the outcome of infants infected with HSV, especially those treated at nonteaching hospitals or with rarer comorbid conditions. The goal of this project was to determine the patient and hospital characteristics associated with worse clinical outcomes in infants under the age of 60 days admitted with HSV disease. We hypothesized that younger infants, infants with a concurrent congenital anomaly, and infants treated at non‐children's hospitals would have worse clinical outcomes. To answer these questions, we used 2003 panel data from the Healthcare Cost and Utilization Project (HCUP) Kids' Inpatient Database (KID), a nationally representative sample of inpatient hospitalizations in the United States.
Methods
Study Population and Data Collection
We conducted a retrospective population cohort study of all infants admitted at 60 days of age who were discharged with a diagnosis of HSV disease between January 1, 2003 and December 31, 2003, using the 2003 KID. The KID is a collaborative project between the Agency for Healthcare Research and Quality AHRQ and 36 states, which includes approximately 2.9 million pediatric discharge records from 3438 hospitals.5 The KID is the only national, all‐payer database of pediatric hospitalizations in the United States.
Patient Eligibility
As in prior studies,611 children were eligible for this project if they were discharged with an International Classification of Disease, ninth edition, Clinical Modification (ICD‐9CM) discharge code of 054.xx (herpes simplex virus), where xx represented any combination of one or two‐digit codes, or 771.2 (neonatal viral infection including HSV). However, the 771.2 code may also contain other perinatal infections of relatively rare frequency, such as toxoplasmosis. Thus, we also performed the same set of analyses on the cohort of children who had an 054.xx code alone. No results presented in this study changed in statistical significance when this smaller cohort of infants was examined.
Data Variables and Outcomes
Outcome Variables
We examined 2 primary clinical outcomes in this study: in‐hospital death and the occurrence of a serious complication. Complications were identified using ICD‐9CM codes from both prior work12 and examination of all diagnosis and procedure codes for eligible infants by the 2 principal investigators (Appendix). These 2 reviewers had to independently agree on the inclusion of an ICD‐9CM code as a complication. In‐hospital deaths were captured through a disposition code of 20 in the KID dataset. Length of stay (LOS) and in‐hospital costs were examined as secondary outcome measures for specific risk factors of interest.
Demographic and Comorbidity Variables
Demographic and comorbidity variables were included in the analyses to control for the increased cost, LOS, or risk of a complication that result from these factors.1315 Demographic information available in the KID included gender, age at admission, race, low birth weight infants, and insurance status. Age at admission was grouped into 4 categories: 07 days, 814 days, 1528 days, and 2960 days. Infants were classified as low birth weight if they had an ICD‐9CM code for a birth weight <2000 g (ICD‐9CM codes 765.01‐07, 765.11‐17, or 765.21‐27). We used the ICD‐9CM codes shown in the Appendix to classify various comorbid conditions. Because of the young age of the cohort, all comorbid conditions consisted of congenital anomalies that were grouped according to the involved organ system. To help classify patients by their illness severity, we used the All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) severity of illness classification for each hospital admission (3M Corporation, St. Paul, MN). The APR‐DRG classification system used discharge diagnoses, procedures, and demographic information to assign patients to 4 severity of illness categories.
Hospital Characteristics
We identified the following hospital characteristics from the KID: total bed size, divided as small, medium, and large; hospital status (children's hospital vs. non‐children's hospital, teaching hospital vs. nonteaching hospital); source of admission (emergency department, clinic, other hospitals); and location (rural vs. urban). Children's hospitals were identified by the AHRQ using information from the National Association of Children's Hospitals and Related Institutions, while teaching hospital status was determined by the presence of an approved residency program and a ratio of full‐time residents to beds of 0.25 or greater.5
Statistical Analysis
All analyses accounted for the complex sampling design with the survey commands included in STATA 9.2 (Statacorp, College Station, TX) and report national estimates from the data available in the 36 surveyed states. Because of the complex sampling design, the Wald test was used to determine significant differences for each outcome in univariable analysis. Variance estimates were reported as standard errors of the mean. We constructed multivariable logistic regression models to assess the adjusted impact of patient and hospital‐level characteristics on each primary outcome measure; ie, in‐hospital death and development of a serious complication. Negative binomial models were used for our secondary outcomes, LOS and costs, because of their rightward skew. Variance estimates for each model accounted for the clustering of data at the hospital level, and data were analyzed as per the latest AHRQ statistical update.16
Results
The 2003 KID identified 1587 hospitalizations for HSV in infants admitted at an age of 60 days or less in the entire United States. These infants had a total hospital cost of $27,147,000. Of the cohort, 10% had a concurrent congenital anomaly. Most infants (73.5%) were admitted within 14 days of birth, and 15.5% were transferred from another hospital. Based on APR‐DRG criteria, 33% of the infants were classified as having a moderate risk of death, 24% as major risk, and 12.2% as extreme risk. The majority of infants were treated at non‐children's hospitals (85.3%) in urban locations (91.5%). The average LOS was 12.0 0.6 days and the average total hospital cost was $17,382 1269. After admission, 267 of the infants, or 16.8%, had at least 1 serious complication. Fifty infants died during the hospitalization included in the KID.
Risk Factor Analysis
Serious Complications
Univariable (Table 1) analysis identified several factors associated with higher rates of serious complications. Younger age at admission was associated with a higher risk of serious complications. This trend was greatest for infants admitted under 14 days of age, of which 20.2% had a serious complication, compared with 10.2% of the infants admitted between 29 and 60 days of age. Infants with any identified congenital anomaly had significantly higher rates of serious complication (41.1% vs. 14.8% for infants without a congenital anomaly). Similar findings were seen with low birth weight infants. Infants who were transferred prior to the hospitalization captured in the KID had a higher complication rate (38.7%) than infants admitted as a routine admission (15.9%) or via the emergency room (8.8%). Among hospital‐level factors, infants admitted to children's or teaching hospitals had higher rates of serious complications, although only the difference between teaching and nonteaching hospitals reached statistical significance (Table 1).
| Patient‐Level Factors | % of Cohort | % with Serious Complication | % Death |
|---|---|---|---|
| |||
| Age at presentation | |||
| 7 days | 58.4 | 21.6* | 4.2* |
| 814 days | 15.1 | 15.8 | 3.6 |
| 1528 days | 16.4 | 9.7 | 2.1 |
| 2960 days | 10.1 | 10.2 | 0 |
| Low birth weight | |||
| Yes | 10.6 | 44.2* | 9.0* |
| No | 89.4 | 14.3 | 2.7 |
| Type of insurance | |||
| Private | 47.4 | 15.6 | 2.1* |
| Medicaid | 49.0 | 19.2 | 4.8 |
| Self pay | 3.6 | 17.0 | 0 |
| Race | |||
| White | 52.8 | 17.7 | 3.5 |
| Black | 18.9 | 17.6 | 4.2 |
| Other | 28.3 | 19.2 | 4.5 |
| Gender | |||
| Female | 45.4 | 15.7 | 2.2 |
| Male | 54.6 | 18.9 | 4.3 |
| Any congenital anomaly | |||
| Yes | 10.0 | 41.1* | 10.4* |
| No | 90.0 | 14.8 | 2.6 |
| Admission type | |||
| Routine | 62.3 | 15.9* | 2.8* |
| Emergency room | 22.2 | 8.8 | 1.1 |
| Transfer from another hospital | 15.5 | 38.7 | 9.6 |
| APR‐DRG risk | |||
| Mild | 3.0 | 0.3* | 0* |
| Moderate | 33.0 | 2.0 | 0.5 |
| Major | 24.0 | 24.7 | 2.3 |
| Extreme | 12.2 | 85.0 | 20.8 |
| Hospital‐level factors | |||
| Children's hospital | |||
| Yes | 14.7 | 27.0 | 6.4 |
| No | 85.3 | 16.3 | 3.1 |
| Teaching hospital | |||
| Yes | 68.4 | 21.3* | 4.3* |
| No | 31.7 | 8.5 | 1.5 |
| Location | |||
| Urban | 91.5 | 18.0* | 3.6 |
| Rural | 8.5 | 9.0 | 1.6 |
| Hospital size | |||
| Small | 14.1 | 19.3 | 4.2 |
| Medium | 25.9 | 14.3 | 3.2 |
| Large | 60.0 | 18.1 | 3.3 |
Many of these factors were independently associated with increased complication rates in multivariable analysis (Table 2). Infants under 7 days of age on admission (odds ratio [OR], 2.68; 95% confidence interval [CI], 1.112.47), low birth weight (OR, 5.17; 95% CI, 2.988.98), and the concurrent presence of a congenital anomaly (OR, 3.09; 95% CI, 1.805.33) were associated with higher odds of a serious complication. Site of care lost its statistical significance once our models adjusted for differences in illness severity. Insurance status, gender, and race were not associated with a change in complication rates for these infants.
| Risk Factor | Serious Complication | Mortality | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| ||||
| Age at admission | ||||
| 7 days | 2.68 | 1.112.47 | 1.63 | 0.347.73 |
| 814 days | 1.22 | 0.403.73 | 2.15 | 0.3612.9 |
| 1428 days | 0.87 | 0.322.37 | Reference* | |
| 2960 days | Reference | |||
| Racial/ethnic status | ||||
| White | Reference | Reference | ||
| Black | 0.90 | 0.451.82 | 1.30 | 0.433.89 |
| Other | 0.99 | 0.571.70 | 1.19 | 0.482.99 |
| Treatment at children's hospital | 2.33 | 0.836.18 | 2.59 | 0.6510.2 |
| Treatment at teaching hospital | 1.71 | 0.943.12 | 1.86 | 0.566.25 |
| Female gender | 0.96 | 0.631.48 | 0.28 | 0.100.82 |
| Medicaid insurance | 1.51 | 0.912.50 | 1.69 | 0.634.53 |
| Transferred from another hospital | 3.76 | 2.036.98 | 3.47 | 1.428.46 |
| Transferred to another hospital | 1.35 | 0.672.73 | ||
| Presence of a congenital anomaly | 3.09 | 1.805.33 | 4.26 | 1.7610.3 |
| Low birth weight infant | 5.17 | 2.988.98 | 5.33 | 1.9015.0 |
Death
Risk factors for higher mortality rates followed similar trends as those for the risk of a serious complication. Younger age at admission, low birth weight status, the presence of a serious complication, admission from another hospital, and treatment at a children's hospital or teaching hospital were all associated with higher mortality rates. In multivariable analysis, the concurrent presence of a congenital anomaly was associated with higher odds of death (OR, 4.26; 95% CI, 1.7610.3). The cause of increased death in infants with congenital anomalies appeared to be a higher rate of serious complications, as including serious complications in the multivariable regression model resulted in the association between congenital anomalies and death losing statistical significance (OR in revised model 1.95; 95% CI, 0.636.05). Site of care again was not associated with differences in mortality after controlling for patient case‐mix.
Concurrent Congenital Anomalies
Based on the higher complication and mortality rates seen in infants with HSV who had a concurrent congenital anomaly, we then investigated how the presence of specific congenital anomalies influenced clinical outcomes, LOS, and total hospital costs with HSV disease. Using the congenital anomaly groups listed in the Appendix, we found that congenital heart disease, central nervous system anomalies, pulmonary anomalies, and gastrointestinal anomalies were each associated with either higher rates of serious complications, longer LOS, or higher total hospital costs compared to infants without congenital anomalies (Table 3). Serious complications occurred most commonly in patients with central nervous system anomalies (55.6%) and congenital heart disease (50.8%), while infants with pulmonary anomalies had the longest LOS (37.1 10.0 days) and highest total hospital costs of all anomaly categories. The types of complications differed by the anomaly group: infants with cardiac and pulmonary anomalies had the highest rates of respiratory complications (45% and 40%, respectively), whereas those with central nervous system anomalies had the highest rates of cardiac complications (51%). Each anomaly class had a similar rate of neurological complications, between 30% and 40%.
| Number* | % With Serious Complication | LOS (days) | Total Hospital Costs (2003 dollars) | |
|---|---|---|---|---|
| ||||
| No congenital anomaly | 1391 | 14.8 | 11.3 0.6 | 15,118 1158 |
| Type of congenital anomaly | ||||
| Congenital heart disease | 73 | 50.8 | 23.5 4.6 | 46,760 9340 |
| Central nervous system anomaly | 31 | 55.6 | 15.4 3.0 | 23,962 5037 |
| Head/neck anomaly | 13 | 40.6 | 11.1 4.6 | 14,132 7860 |
| Pulmonary anomaly | 13 | 34.1 | 37.1 10.0 | 67,234 21,002 |
| Gastrointestinal anomaly | 20 | 33.5 | 21.6 4.9 | 41,207 13,878 |
| Genitourinary anomaly | 19 | 24.1 | 11.0 2.5 | 10,906 1890 |
| Musculoskeletal anomaly | ||||
| Genetic anomaly | 18 | 10.2 | 12.2 2.4 | 15,990 3808 |
Site of Care
Finally, we examined the LOS and costs of receiving care at a children's hospital. The data shown in Tables 1 and 2 suggest that receiving treatment at a children's hospital does not result in improved clinical outcomes for infants admitted with HSV. One potential advantage, though, is improved efficiency of care, which would result in a shorter LOS or lower costs. Using negative binomial multivariable regression models to account for differences in patient characteristics, regional variation, and insurance status, treatment at a children's hospital was associated with an 18% shorter LOS (95% CI, 1%34%) compared to non‐children's hospitals after accounting for the generally sicker infants treated at children's hospitals. Children's hospitals, though, were more expensive than non‐children's hospitals (increase of $642 per day; 95% CI, $2321052). These results remained consistent when we omitted transferred patients from the model, instead of controlling for them in the analysis.
Conclusions
There has been little prior information to guide practitioners and parents about factors that potentially influence clinical outcome of infants hospitalized with HSV in non‐children's hospitals, although over 80% of infants are managed at non‐children's hospitals. These studies also did not have the power to characterize the risk of poor clinical outcome associated with rarer clinical factors.1, 2, 6 This study, using nationally representative data, found that these rarer clinical factors and site of care may influence the outcomes of infants hospitalized with HSV, albeit in different methods. Younger age at admission and a coexisting congenital anomaly remained statistically significant predictors of worse clinical outcomes after controlling for various patient and hospital factors. Not all congenital anomalies increased the risk of death or serious complications; rather, anomalies that affected either the cardiopulmonary system or the central nervous system appeared to result in the highest increases in risk. This study also found that treatment of infants with HSV at a children's hospital was associated with a 28% shorter LOS after accounting for the sicker patients cared for by children's hospitals. This finding is in contrast to prior studies of common pediatric conditions, where there were no differences in the LOS between children's and non‐children's hospitals,17, 18 and severe sepsis, where children's hospitals had longer LOSs.19 These results confirm the importance of specific risk factors in predicting the likelihood that an infant admitted with HSV may have a poor clinical outcome. Also, these results emphasize the differences in outcomes that may occur at different types of hospitals.
This study is the first to find that certain congenital anomalies or conditions may be associated with worse clinical outcomes from HSV. There is little information in the literature to explain these findings. Those anomalies that affect the cardiopulmonary or central nervous system may either worsen the symptoms of HSV or predispose infants to have a serious complication, such as shock or respiratory failure. This finding would be similar to the increased risk of serious complications seen in infants with congenital heart disease who contract respiratory syncytial virus20 or infants with genetic syndromes who undergo heart surgery.21 Alternatively, because we do not have information on do‐not‐resuscitate status, the presence of one of these congenital anomalies may result in more withdrawal of care when an infant is infected with HSV and has a serious complication; the LOS of these children may not reflect these decisions because the decision to withdrawal care may only occur after the child's condition worsens significantly, which may happen any time during the disease course. However, this theory is less likely because we failed to find similar results with other congenital anomalies such as genetic or chromosomal syndromes. Further examination of these infants and their overall response to insults such as HSV is needed to understand how these anomalies influence the outcomes of a serious, unrelated illness.
Age upon admission was another important predictor of poor outcomes when analyzed in univariable or multivariable analysis. This result is consistent with prior work,14 which suggests that younger children are more likely to be hospitalized with either congenitally acquired HSV or systemic disease. The information contained in the KID does not allow us to determine whether young age is a risk factor for poor outcome irrespective of the clinical presentation of HSV, or whether age serves as a proxy for the appearance of more severe clinical disease. This effect of age remained present even after controlling for the higher risk of a serious complication and death in low birth weight infants. There are limited data that suggest that premature birth is an independent risk factor for worse outcomes associated with perinatal or congenital infection; 1 previous case study of Enterobacter sakazakii infections found a higher fatality rate for premature infants compared to term infants.22 This study supports these findings.
This study found that treatment at a children's hospital resulted in a 28% shorter LOS without a statistically significant difference in clinical outcomes after controlling for case‐mix differences. This finding is in contrast to prior studies of common pediatric conditions17, 18 and severe sepsis.19 There are several potential explanations for the difference in findings. For common pediatric conditions, there may be fewer variations in treatment style and less need for new diagnostic modalities that are more available at academic centers. For HSV disease, though, children's hospitals may also be more likely than non‐children's hospitals to perform polymerase‐chain reaction (PCR) testing for the diagnosis of perinatally acquired HSV, correctly identify the disorder, or receive the test results in a timely fashion. Pediatric subspecialists, such as infectious disease physicians or neurologists, are also likely to be more available at children's hospitals than at other centers. While the role of subspecialty consultation in improving outcomes for neonates with HSV is not known, improved outcomes at children's hospitals has been described for other serious conditions such as splenic injuries.23 Children's hospitals had higher daily costs than non‐children's hospitals, as has been found in other work.17, 19 Children's hospitals may be treating sicker patients, for whom we are unable to adequately adjust for their illness severity with hospital administrative data.17, 19 Also, there may be a greater use of medical tests and treatments that increase the costs of care. These costs do not include indirect costs to the families such as loss of work and travel costs. In light of the shorter LOS in children's hospitals, policy makers will need to balance the potentially higher daily costs of care with more efficient management of the disease process.
Because this study used hospital administrative records, there are a few limitations. We used ICD‐9CM diagnosis codes to identify patients, congenital anomalies, and complications. The diagnosis of some infants with HSV or less significant congenital anomalies could have been missed because clinicians either overlooked the disease or did not make the diagnosis before discharge. This form of spectrum bias would likely miss the infants with the least severe disease and make it more difficult to find the results that we found in this study.24 Prior work successfully used and validated similar ICD‐9CM codes to identify HSV cases among the different types of hospitals included in the KID.611 Our study design estimated 1587 cases of neonatal HSV in 2003. A prospective study of maternal serologic and virologic status during pregnancy estimated 480 to 2160 new cases of neonatal HSV per year.25 Thus, while miscoding is a potential limitation to our study, the overall numbers of patients in this study were similar to past annual estimates. One potential area of miscounting, though, was the inability of the KID to link the records of 16% of the identified infants with HSV whose care was transferred between hospitals. These infants may result in misleading LOS or cost information: lower for the transferring hospital, because they only kept the child a short period of time, or lower for the accepting hospital, as some of the total hospital stay is not accounted for in the KID. We accounted for this issue in 2 ways. First, we included a variable for being transferred in the multivariable models, and found no difference in any results when we omitted these patients from the analysis. Second, we performed a univariable analysis stratified by transfer status, which did not differ substantially from our main model for most variables. Accurate linkage of all the hospital records for an infant's hospital course, likely only through a mandatory reporting system for infant HSV, would help confirm the associations we identified in this study.
In conclusion, infants with congenital anomalies should be closely monitored for the development of serious complications associated with HSV, particularly those infants with congenital heart disease, pulmonary anomalies, or central nervous system anomalies. Closer investigation of the care practices that children's hospitals use in the management of infants with HSV is needed to improve the efficiency of care delivered to these infants, as HSV disease remains a significant public health problem.
Herpes simplex virus (HSV) is a significant cause of pediatric hospitalization, morbidity and mortality, particularly in infants under 60 days of age, where HSV can present as meningoencephalitis, skin disease, or sepsis.14 Most prior studies use data from registries taken from single centers or a restricted group of hospitals. Thus, there is a paucity of recent, nationally‐representative information about the outcome of infants infected with HSV, especially those treated at nonteaching hospitals or with rarer comorbid conditions. The goal of this project was to determine the patient and hospital characteristics associated with worse clinical outcomes in infants under the age of 60 days admitted with HSV disease. We hypothesized that younger infants, infants with a concurrent congenital anomaly, and infants treated at non‐children's hospitals would have worse clinical outcomes. To answer these questions, we used 2003 panel data from the Healthcare Cost and Utilization Project (HCUP) Kids' Inpatient Database (KID), a nationally representative sample of inpatient hospitalizations in the United States.
Methods
Study Population and Data Collection
We conducted a retrospective population cohort study of all infants admitted at 60 days of age who were discharged with a diagnosis of HSV disease between January 1, 2003 and December 31, 2003, using the 2003 KID. The KID is a collaborative project between the Agency for Healthcare Research and Quality AHRQ and 36 states, which includes approximately 2.9 million pediatric discharge records from 3438 hospitals.5 The KID is the only national, all‐payer database of pediatric hospitalizations in the United States.
Patient Eligibility
As in prior studies,611 children were eligible for this project if they were discharged with an International Classification of Disease, ninth edition, Clinical Modification (ICD‐9CM) discharge code of 054.xx (herpes simplex virus), where xx represented any combination of one or two‐digit codes, or 771.2 (neonatal viral infection including HSV). However, the 771.2 code may also contain other perinatal infections of relatively rare frequency, such as toxoplasmosis. Thus, we also performed the same set of analyses on the cohort of children who had an 054.xx code alone. No results presented in this study changed in statistical significance when this smaller cohort of infants was examined.
Data Variables and Outcomes
Outcome Variables
We examined 2 primary clinical outcomes in this study: in‐hospital death and the occurrence of a serious complication. Complications were identified using ICD‐9CM codes from both prior work12 and examination of all diagnosis and procedure codes for eligible infants by the 2 principal investigators (Appendix). These 2 reviewers had to independently agree on the inclusion of an ICD‐9CM code as a complication. In‐hospital deaths were captured through a disposition code of 20 in the KID dataset. Length of stay (LOS) and in‐hospital costs were examined as secondary outcome measures for specific risk factors of interest.
Demographic and Comorbidity Variables
Demographic and comorbidity variables were included in the analyses to control for the increased cost, LOS, or risk of a complication that result from these factors.1315 Demographic information available in the KID included gender, age at admission, race, low birth weight infants, and insurance status. Age at admission was grouped into 4 categories: 07 days, 814 days, 1528 days, and 2960 days. Infants were classified as low birth weight if they had an ICD‐9CM code for a birth weight <2000 g (ICD‐9CM codes 765.01‐07, 765.11‐17, or 765.21‐27). We used the ICD‐9CM codes shown in the Appendix to classify various comorbid conditions. Because of the young age of the cohort, all comorbid conditions consisted of congenital anomalies that were grouped according to the involved organ system. To help classify patients by their illness severity, we used the All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) severity of illness classification for each hospital admission (3M Corporation, St. Paul, MN). The APR‐DRG classification system used discharge diagnoses, procedures, and demographic information to assign patients to 4 severity of illness categories.
Hospital Characteristics
We identified the following hospital characteristics from the KID: total bed size, divided as small, medium, and large; hospital status (children's hospital vs. non‐children's hospital, teaching hospital vs. nonteaching hospital); source of admission (emergency department, clinic, other hospitals); and location (rural vs. urban). Children's hospitals were identified by the AHRQ using information from the National Association of Children's Hospitals and Related Institutions, while teaching hospital status was determined by the presence of an approved residency program and a ratio of full‐time residents to beds of 0.25 or greater.5
Statistical Analysis
All analyses accounted for the complex sampling design with the survey commands included in STATA 9.2 (Statacorp, College Station, TX) and report national estimates from the data available in the 36 surveyed states. Because of the complex sampling design, the Wald test was used to determine significant differences for each outcome in univariable analysis. Variance estimates were reported as standard errors of the mean. We constructed multivariable logistic regression models to assess the adjusted impact of patient and hospital‐level characteristics on each primary outcome measure; ie, in‐hospital death and development of a serious complication. Negative binomial models were used for our secondary outcomes, LOS and costs, because of their rightward skew. Variance estimates for each model accounted for the clustering of data at the hospital level, and data were analyzed as per the latest AHRQ statistical update.16
Results
The 2003 KID identified 1587 hospitalizations for HSV in infants admitted at an age of 60 days or less in the entire United States. These infants had a total hospital cost of $27,147,000. Of the cohort, 10% had a concurrent congenital anomaly. Most infants (73.5%) were admitted within 14 days of birth, and 15.5% were transferred from another hospital. Based on APR‐DRG criteria, 33% of the infants were classified as having a moderate risk of death, 24% as major risk, and 12.2% as extreme risk. The majority of infants were treated at non‐children's hospitals (85.3%) in urban locations (91.5%). The average LOS was 12.0 0.6 days and the average total hospital cost was $17,382 1269. After admission, 267 of the infants, or 16.8%, had at least 1 serious complication. Fifty infants died during the hospitalization included in the KID.
Risk Factor Analysis
Serious Complications
Univariable (Table 1) analysis identified several factors associated with higher rates of serious complications. Younger age at admission was associated with a higher risk of serious complications. This trend was greatest for infants admitted under 14 days of age, of which 20.2% had a serious complication, compared with 10.2% of the infants admitted between 29 and 60 days of age. Infants with any identified congenital anomaly had significantly higher rates of serious complication (41.1% vs. 14.8% for infants without a congenital anomaly). Similar findings were seen with low birth weight infants. Infants who were transferred prior to the hospitalization captured in the KID had a higher complication rate (38.7%) than infants admitted as a routine admission (15.9%) or via the emergency room (8.8%). Among hospital‐level factors, infants admitted to children's or teaching hospitals had higher rates of serious complications, although only the difference between teaching and nonteaching hospitals reached statistical significance (Table 1).
| Patient‐Level Factors | % of Cohort | % with Serious Complication | % Death |
|---|---|---|---|
| |||
| Age at presentation | |||
| 7 days | 58.4 | 21.6* | 4.2* |
| 814 days | 15.1 | 15.8 | 3.6 |
| 1528 days | 16.4 | 9.7 | 2.1 |
| 2960 days | 10.1 | 10.2 | 0 |
| Low birth weight | |||
| Yes | 10.6 | 44.2* | 9.0* |
| No | 89.4 | 14.3 | 2.7 |
| Type of insurance | |||
| Private | 47.4 | 15.6 | 2.1* |
| Medicaid | 49.0 | 19.2 | 4.8 |
| Self pay | 3.6 | 17.0 | 0 |
| Race | |||
| White | 52.8 | 17.7 | 3.5 |
| Black | 18.9 | 17.6 | 4.2 |
| Other | 28.3 | 19.2 | 4.5 |
| Gender | |||
| Female | 45.4 | 15.7 | 2.2 |
| Male | 54.6 | 18.9 | 4.3 |
| Any congenital anomaly | |||
| Yes | 10.0 | 41.1* | 10.4* |
| No | 90.0 | 14.8 | 2.6 |
| Admission type | |||
| Routine | 62.3 | 15.9* | 2.8* |
| Emergency room | 22.2 | 8.8 | 1.1 |
| Transfer from another hospital | 15.5 | 38.7 | 9.6 |
| APR‐DRG risk | |||
| Mild | 3.0 | 0.3* | 0* |
| Moderate | 33.0 | 2.0 | 0.5 |
| Major | 24.0 | 24.7 | 2.3 |
| Extreme | 12.2 | 85.0 | 20.8 |
| Hospital‐level factors | |||
| Children's hospital | |||
| Yes | 14.7 | 27.0 | 6.4 |
| No | 85.3 | 16.3 | 3.1 |
| Teaching hospital | |||
| Yes | 68.4 | 21.3* | 4.3* |
| No | 31.7 | 8.5 | 1.5 |
| Location | |||
| Urban | 91.5 | 18.0* | 3.6 |
| Rural | 8.5 | 9.0 | 1.6 |
| Hospital size | |||
| Small | 14.1 | 19.3 | 4.2 |
| Medium | 25.9 | 14.3 | 3.2 |
| Large | 60.0 | 18.1 | 3.3 |
Many of these factors were independently associated with increased complication rates in multivariable analysis (Table 2). Infants under 7 days of age on admission (odds ratio [OR], 2.68; 95% confidence interval [CI], 1.112.47), low birth weight (OR, 5.17; 95% CI, 2.988.98), and the concurrent presence of a congenital anomaly (OR, 3.09; 95% CI, 1.805.33) were associated with higher odds of a serious complication. Site of care lost its statistical significance once our models adjusted for differences in illness severity. Insurance status, gender, and race were not associated with a change in complication rates for these infants.
| Risk Factor | Serious Complication | Mortality | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| ||||
| Age at admission | ||||
| 7 days | 2.68 | 1.112.47 | 1.63 | 0.347.73 |
| 814 days | 1.22 | 0.403.73 | 2.15 | 0.3612.9 |
| 1428 days | 0.87 | 0.322.37 | Reference* | |
| 2960 days | Reference | |||
| Racial/ethnic status | ||||
| White | Reference | Reference | ||
| Black | 0.90 | 0.451.82 | 1.30 | 0.433.89 |
| Other | 0.99 | 0.571.70 | 1.19 | 0.482.99 |
| Treatment at children's hospital | 2.33 | 0.836.18 | 2.59 | 0.6510.2 |
| Treatment at teaching hospital | 1.71 | 0.943.12 | 1.86 | 0.566.25 |
| Female gender | 0.96 | 0.631.48 | 0.28 | 0.100.82 |
| Medicaid insurance | 1.51 | 0.912.50 | 1.69 | 0.634.53 |
| Transferred from another hospital | 3.76 | 2.036.98 | 3.47 | 1.428.46 |
| Transferred to another hospital | 1.35 | 0.672.73 | ||
| Presence of a congenital anomaly | 3.09 | 1.805.33 | 4.26 | 1.7610.3 |
| Low birth weight infant | 5.17 | 2.988.98 | 5.33 | 1.9015.0 |
Death
Risk factors for higher mortality rates followed similar trends as those for the risk of a serious complication. Younger age at admission, low birth weight status, the presence of a serious complication, admission from another hospital, and treatment at a children's hospital or teaching hospital were all associated with higher mortality rates. In multivariable analysis, the concurrent presence of a congenital anomaly was associated with higher odds of death (OR, 4.26; 95% CI, 1.7610.3). The cause of increased death in infants with congenital anomalies appeared to be a higher rate of serious complications, as including serious complications in the multivariable regression model resulted in the association between congenital anomalies and death losing statistical significance (OR in revised model 1.95; 95% CI, 0.636.05). Site of care again was not associated with differences in mortality after controlling for patient case‐mix.
Concurrent Congenital Anomalies
Based on the higher complication and mortality rates seen in infants with HSV who had a concurrent congenital anomaly, we then investigated how the presence of specific congenital anomalies influenced clinical outcomes, LOS, and total hospital costs with HSV disease. Using the congenital anomaly groups listed in the Appendix, we found that congenital heart disease, central nervous system anomalies, pulmonary anomalies, and gastrointestinal anomalies were each associated with either higher rates of serious complications, longer LOS, or higher total hospital costs compared to infants without congenital anomalies (Table 3). Serious complications occurred most commonly in patients with central nervous system anomalies (55.6%) and congenital heart disease (50.8%), while infants with pulmonary anomalies had the longest LOS (37.1 10.0 days) and highest total hospital costs of all anomaly categories. The types of complications differed by the anomaly group: infants with cardiac and pulmonary anomalies had the highest rates of respiratory complications (45% and 40%, respectively), whereas those with central nervous system anomalies had the highest rates of cardiac complications (51%). Each anomaly class had a similar rate of neurological complications, between 30% and 40%.
| Number* | % With Serious Complication | LOS (days) | Total Hospital Costs (2003 dollars) | |
|---|---|---|---|---|
| ||||
| No congenital anomaly | 1391 | 14.8 | 11.3 0.6 | 15,118 1158 |
| Type of congenital anomaly | ||||
| Congenital heart disease | 73 | 50.8 | 23.5 4.6 | 46,760 9340 |
| Central nervous system anomaly | 31 | 55.6 | 15.4 3.0 | 23,962 5037 |
| Head/neck anomaly | 13 | 40.6 | 11.1 4.6 | 14,132 7860 |
| Pulmonary anomaly | 13 | 34.1 | 37.1 10.0 | 67,234 21,002 |
| Gastrointestinal anomaly | 20 | 33.5 | 21.6 4.9 | 41,207 13,878 |
| Genitourinary anomaly | 19 | 24.1 | 11.0 2.5 | 10,906 1890 |
| Musculoskeletal anomaly | ||||
| Genetic anomaly | 18 | 10.2 | 12.2 2.4 | 15,990 3808 |
Site of Care
Finally, we examined the LOS and costs of receiving care at a children's hospital. The data shown in Tables 1 and 2 suggest that receiving treatment at a children's hospital does not result in improved clinical outcomes for infants admitted with HSV. One potential advantage, though, is improved efficiency of care, which would result in a shorter LOS or lower costs. Using negative binomial multivariable regression models to account for differences in patient characteristics, regional variation, and insurance status, treatment at a children's hospital was associated with an 18% shorter LOS (95% CI, 1%34%) compared to non‐children's hospitals after accounting for the generally sicker infants treated at children's hospitals. Children's hospitals, though, were more expensive than non‐children's hospitals (increase of $642 per day; 95% CI, $2321052). These results remained consistent when we omitted transferred patients from the model, instead of controlling for them in the analysis.
Conclusions
There has been little prior information to guide practitioners and parents about factors that potentially influence clinical outcome of infants hospitalized with HSV in non‐children's hospitals, although over 80% of infants are managed at non‐children's hospitals. These studies also did not have the power to characterize the risk of poor clinical outcome associated with rarer clinical factors.1, 2, 6 This study, using nationally representative data, found that these rarer clinical factors and site of care may influence the outcomes of infants hospitalized with HSV, albeit in different methods. Younger age at admission and a coexisting congenital anomaly remained statistically significant predictors of worse clinical outcomes after controlling for various patient and hospital factors. Not all congenital anomalies increased the risk of death or serious complications; rather, anomalies that affected either the cardiopulmonary system or the central nervous system appeared to result in the highest increases in risk. This study also found that treatment of infants with HSV at a children's hospital was associated with a 28% shorter LOS after accounting for the sicker patients cared for by children's hospitals. This finding is in contrast to prior studies of common pediatric conditions, where there were no differences in the LOS between children's and non‐children's hospitals,17, 18 and severe sepsis, where children's hospitals had longer LOSs.19 These results confirm the importance of specific risk factors in predicting the likelihood that an infant admitted with HSV may have a poor clinical outcome. Also, these results emphasize the differences in outcomes that may occur at different types of hospitals.
This study is the first to find that certain congenital anomalies or conditions may be associated with worse clinical outcomes from HSV. There is little information in the literature to explain these findings. Those anomalies that affect the cardiopulmonary or central nervous system may either worsen the symptoms of HSV or predispose infants to have a serious complication, such as shock or respiratory failure. This finding would be similar to the increased risk of serious complications seen in infants with congenital heart disease who contract respiratory syncytial virus20 or infants with genetic syndromes who undergo heart surgery.21 Alternatively, because we do not have information on do‐not‐resuscitate status, the presence of one of these congenital anomalies may result in more withdrawal of care when an infant is infected with HSV and has a serious complication; the LOS of these children may not reflect these decisions because the decision to withdrawal care may only occur after the child's condition worsens significantly, which may happen any time during the disease course. However, this theory is less likely because we failed to find similar results with other congenital anomalies such as genetic or chromosomal syndromes. Further examination of these infants and their overall response to insults such as HSV is needed to understand how these anomalies influence the outcomes of a serious, unrelated illness.
Age upon admission was another important predictor of poor outcomes when analyzed in univariable or multivariable analysis. This result is consistent with prior work,14 which suggests that younger children are more likely to be hospitalized with either congenitally acquired HSV or systemic disease. The information contained in the KID does not allow us to determine whether young age is a risk factor for poor outcome irrespective of the clinical presentation of HSV, or whether age serves as a proxy for the appearance of more severe clinical disease. This effect of age remained present even after controlling for the higher risk of a serious complication and death in low birth weight infants. There are limited data that suggest that premature birth is an independent risk factor for worse outcomes associated with perinatal or congenital infection; 1 previous case study of Enterobacter sakazakii infections found a higher fatality rate for premature infants compared to term infants.22 This study supports these findings.
This study found that treatment at a children's hospital resulted in a 28% shorter LOS without a statistically significant difference in clinical outcomes after controlling for case‐mix differences. This finding is in contrast to prior studies of common pediatric conditions17, 18 and severe sepsis.19 There are several potential explanations for the difference in findings. For common pediatric conditions, there may be fewer variations in treatment style and less need for new diagnostic modalities that are more available at academic centers. For HSV disease, though, children's hospitals may also be more likely than non‐children's hospitals to perform polymerase‐chain reaction (PCR) testing for the diagnosis of perinatally acquired HSV, correctly identify the disorder, or receive the test results in a timely fashion. Pediatric subspecialists, such as infectious disease physicians or neurologists, are also likely to be more available at children's hospitals than at other centers. While the role of subspecialty consultation in improving outcomes for neonates with HSV is not known, improved outcomes at children's hospitals has been described for other serious conditions such as splenic injuries.23 Children's hospitals had higher daily costs than non‐children's hospitals, as has been found in other work.17, 19 Children's hospitals may be treating sicker patients, for whom we are unable to adequately adjust for their illness severity with hospital administrative data.17, 19 Also, there may be a greater use of medical tests and treatments that increase the costs of care. These costs do not include indirect costs to the families such as loss of work and travel costs. In light of the shorter LOS in children's hospitals, policy makers will need to balance the potentially higher daily costs of care with more efficient management of the disease process.
Because this study used hospital administrative records, there are a few limitations. We used ICD‐9CM diagnosis codes to identify patients, congenital anomalies, and complications. The diagnosis of some infants with HSV or less significant congenital anomalies could have been missed because clinicians either overlooked the disease or did not make the diagnosis before discharge. This form of spectrum bias would likely miss the infants with the least severe disease and make it more difficult to find the results that we found in this study.24 Prior work successfully used and validated similar ICD‐9CM codes to identify HSV cases among the different types of hospitals included in the KID.611 Our study design estimated 1587 cases of neonatal HSV in 2003. A prospective study of maternal serologic and virologic status during pregnancy estimated 480 to 2160 new cases of neonatal HSV per year.25 Thus, while miscoding is a potential limitation to our study, the overall numbers of patients in this study were similar to past annual estimates. One potential area of miscounting, though, was the inability of the KID to link the records of 16% of the identified infants with HSV whose care was transferred between hospitals. These infants may result in misleading LOS or cost information: lower for the transferring hospital, because they only kept the child a short period of time, or lower for the accepting hospital, as some of the total hospital stay is not accounted for in the KID. We accounted for this issue in 2 ways. First, we included a variable for being transferred in the multivariable models, and found no difference in any results when we omitted these patients from the analysis. Second, we performed a univariable analysis stratified by transfer status, which did not differ substantially from our main model for most variables. Accurate linkage of all the hospital records for an infant's hospital course, likely only through a mandatory reporting system for infant HSV, would help confirm the associations we identified in this study.
In conclusion, infants with congenital anomalies should be closely monitored for the development of serious complications associated with HSV, particularly those infants with congenital heart disease, pulmonary anomalies, or central nervous system anomalies. Closer investigation of the care practices that children's hospitals use in the management of infants with HSV is needed to improve the efficiency of care delivered to these infants, as HSV disease remains a significant public health problem.
- ,,, et al.Natural history of neonatal herpes simplex virus infections in the acyclovir era.Pediatrics.2001;108:223–229.
- ,,.Herpes simplex viruses.Clin Infect Dis.1998;26:541–553.
- ,,.Herpes simplex virus infections. In: Remington JS, Wilson CB, Baker CJ, editors.Infectious Diseases of the Fetus and Newborn Infant.5th ed.Philadelphia, PA:W.B. Saunders;2001. p425–446.
- ,,, et al.Changing presentation of herpes simplex virus infection in neonates.J Infect Dis.1988;158:109–116.
- Design of the HCUP Kids' Inpatient Database (KID), 2003. Healthcare Cost and Utilization Project (HCUP).Rockville, MD:Agency for Healthcare Research and Quality;2003. Revised January 30, 2006. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/KID_2003_Design_Edited_013006.pdf. Accessed October 2009.
- ,,.Incidence of neonatal herpes simplex virus infections in a managed‐care population.Sex Transm Dis.2007;34:704–708.
- ,,, et al.Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate.Am J Obstet Gynecol.2006;194:408–414.
- ,,, et al.The estimated economic burden of genital herpes in the united states.BMC Infect Dis.2001;1:5.
- ,,, et al.Accuracy of obstetric diagnoses and procedures in hospital discharge data.Am J Obstet Gynecol.2006;194:992–1001.
- ,,, et al.The epidemiology of neonatal herpes simplex virus infections in California from 1985 to 1995.J Infect Dis.1999;180:199–202.
- ,,.Medical care expenditures for genital herpes in the United States.Sex Transm Dis.2000;27:32–38.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,, et al.The importance of comorbidities in explaining differences in patient costs.Med Care.1996;34:767–782.
- ,,, et al.Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population‐based study.Arch Pediatr Adolesc Med.1997;151:1096–1103.
- ,,.The influence of chronic disease on resource utilization in common acute pediatric conditions. Financial concerns for children's hospitals.Arch Pediatr Adolesc Med.1999;153:169–179.
- Health Care Cost and Utility Project.Calculating Kids' Inpatient Database (KID) Variances. December 16, 2005. Methods Series Report # 2005‐5.Rockville, MD:Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/CalculatingKIDVariances.pdf. Accessed October2009.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Length of stay for common pediatric conditions: teaching versus nonteaching hospitals.Pediatrics.2003;112:278–281.
- ,,.Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- .Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection.J Pediatr.2003;143:S112–S117.
- ,,, et al.Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery.J Thorac Cardiovasc Surg.2007;133:1344–1353,1353,e1341–e1343.
- .Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature.Medicine.2001;80:113–122.
- ,,, et al.Hospital characteristics associated with the management of pediatric splenic injuries.JAMA.2005;294:2611–2617.
- ,.Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation.Ann Intern Med.2002;137:598–602.
- ,,, et al.Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant.JAMA.2003;289:203–209.
- ,,, et al.Natural history of neonatal herpes simplex virus infections in the acyclovir era.Pediatrics.2001;108:223–229.
- ,,.Herpes simplex viruses.Clin Infect Dis.1998;26:541–553.
- ,,.Herpes simplex virus infections. In: Remington JS, Wilson CB, Baker CJ, editors.Infectious Diseases of the Fetus and Newborn Infant.5th ed.Philadelphia, PA:W.B. Saunders;2001. p425–446.
- ,,, et al.Changing presentation of herpes simplex virus infection in neonates.J Infect Dis.1988;158:109–116.
- Design of the HCUP Kids' Inpatient Database (KID), 2003. Healthcare Cost and Utilization Project (HCUP).Rockville, MD:Agency for Healthcare Research and Quality;2003. Revised January 30, 2006. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/KID_2003_Design_Edited_013006.pdf. Accessed October 2009.
- ,,.Incidence of neonatal herpes simplex virus infections in a managed‐care population.Sex Transm Dis.2007;34:704–708.
- ,,, et al.Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate.Am J Obstet Gynecol.2006;194:408–414.
- ,,, et al.The estimated economic burden of genital herpes in the united states.BMC Infect Dis.2001;1:5.
- ,,, et al.Accuracy of obstetric diagnoses and procedures in hospital discharge data.Am J Obstet Gynecol.2006;194:992–1001.
- ,,, et al.The epidemiology of neonatal herpes simplex virus infections in California from 1985 to 1995.J Infect Dis.1999;180:199–202.
- ,,.Medical care expenditures for genital herpes in the United States.Sex Transm Dis.2000;27:32–38.
- ,,, et al.The epidemiology of sepsis in the United States from 1979 through 2000.N Engl J Med.2003;348:1546–1554.
- ,,, et al.The importance of comorbidities in explaining differences in patient costs.Med Care.1996;34:767–782.
- ,,, et al.Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population‐based study.Arch Pediatr Adolesc Med.1997;151:1096–1103.
- ,,.The influence of chronic disease on resource utilization in common acute pediatric conditions. Financial concerns for children's hospitals.Arch Pediatr Adolesc Med.1999;153:169–179.
- Health Care Cost and Utility Project.Calculating Kids' Inpatient Database (KID) Variances. December 16, 2005. Methods Series Report # 2005‐5.Rockville, MD:Agency for Healthcare Research and Quality. Available at: http://www.hcup‐us.ahrq.gov/db/nation/kid/reports/CalculatingKIDVariances.pdf. Accessed October2009.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,.Length of stay for common pediatric conditions: teaching versus nonteaching hospitals.Pediatrics.2003;112:278–281.
- ,,.Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- .Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection.J Pediatr.2003;143:S112–S117.
- ,,, et al.Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery.J Thorac Cardiovasc Surg.2007;133:1344–1353,1353,e1341–e1343.
- .Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature.Medicine.2001;80:113–122.
- ,,, et al.Hospital characteristics associated with the management of pediatric splenic injuries.JAMA.2005;294:2611–2617.
- ,.Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation.Ann Intern Med.2002;137:598–602.
- ,,, et al.Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant.JAMA.2003;289:203–209.
Copyright © 2010 Society of Hospital Medicine
Ancillary Testing for Rotavirus
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
Copyright © 2009 Society of Hospital Medicine