User login
Evidence for Thromboembolism Prophylaxis
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
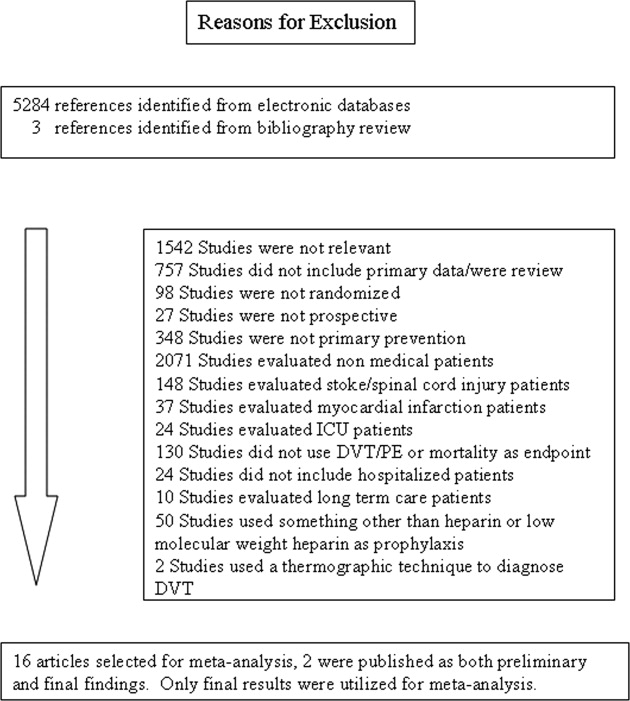
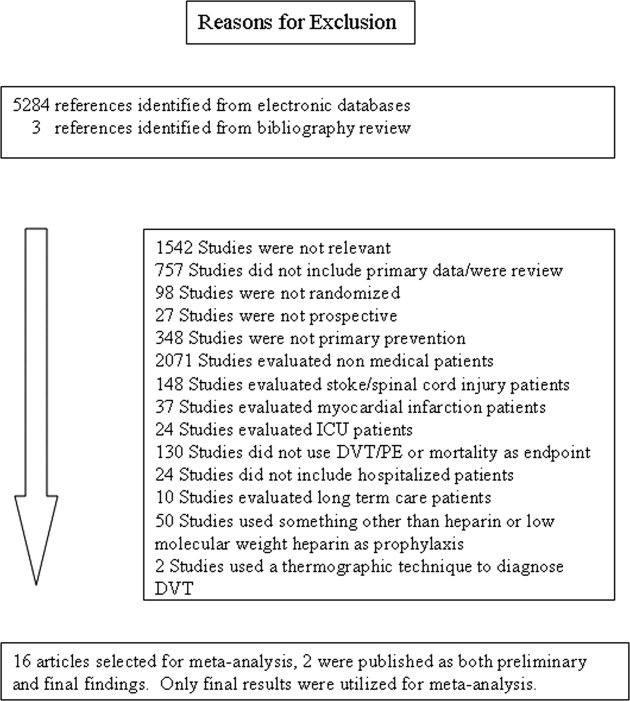
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).
Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
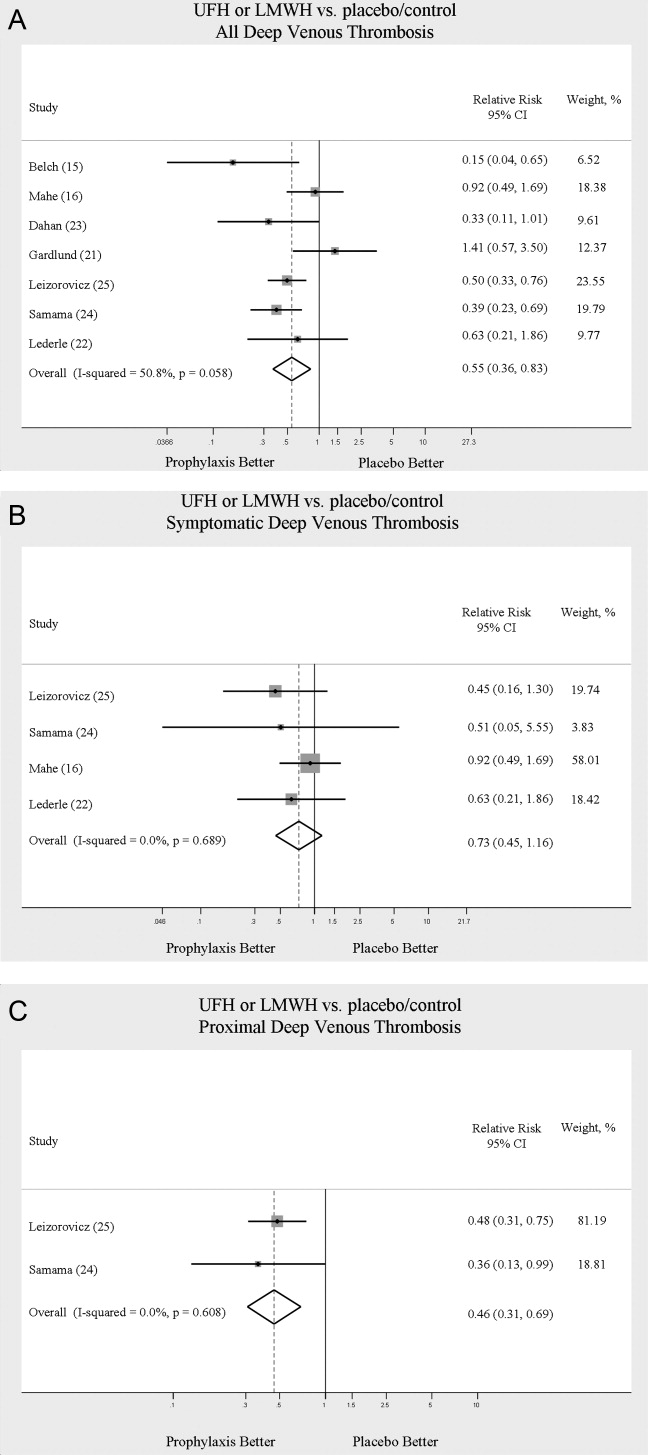
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).
PE
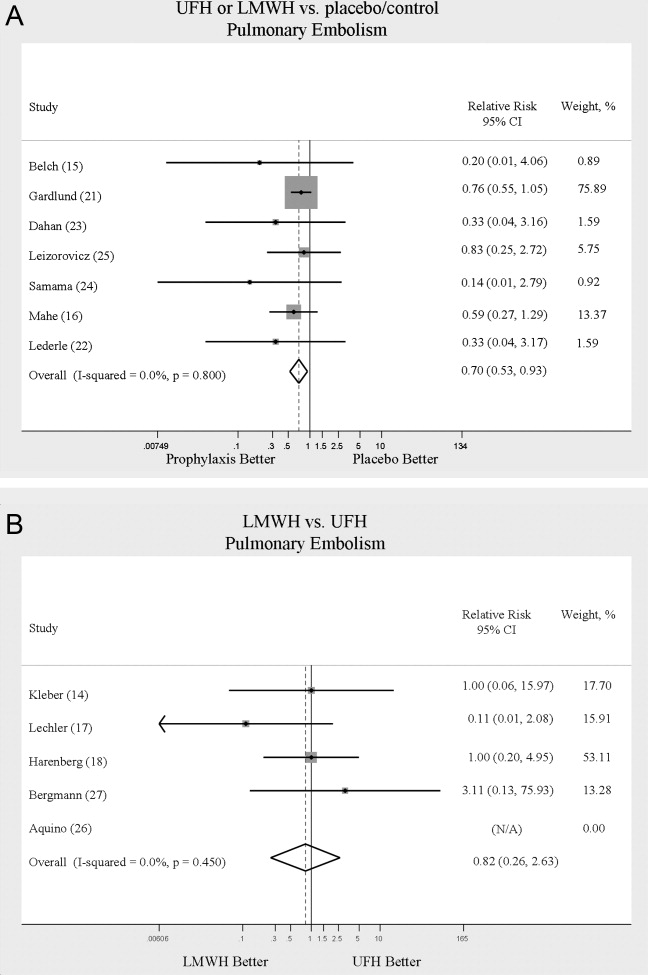
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).
Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
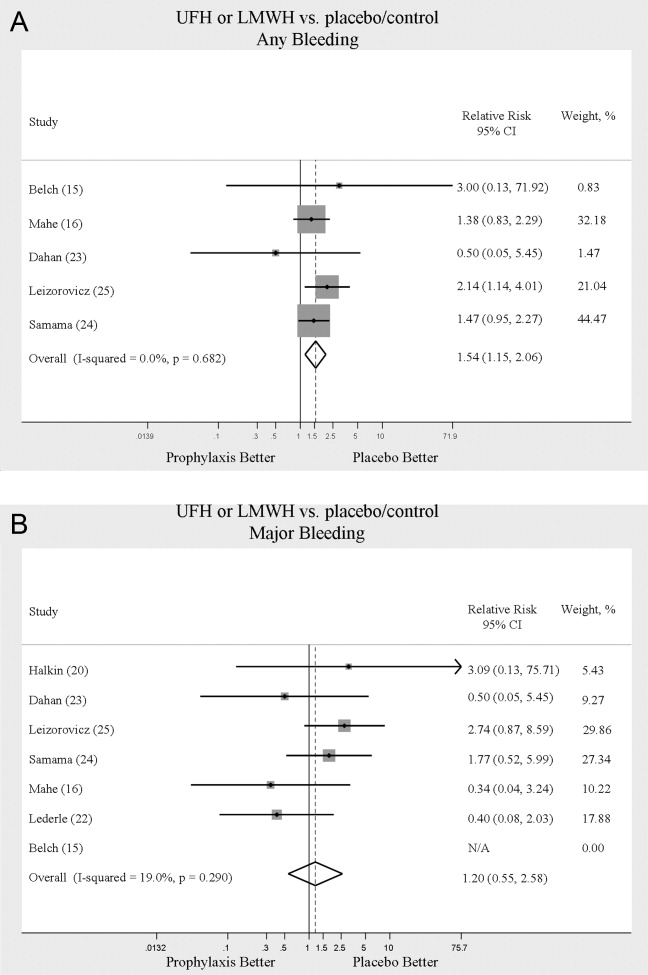
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).
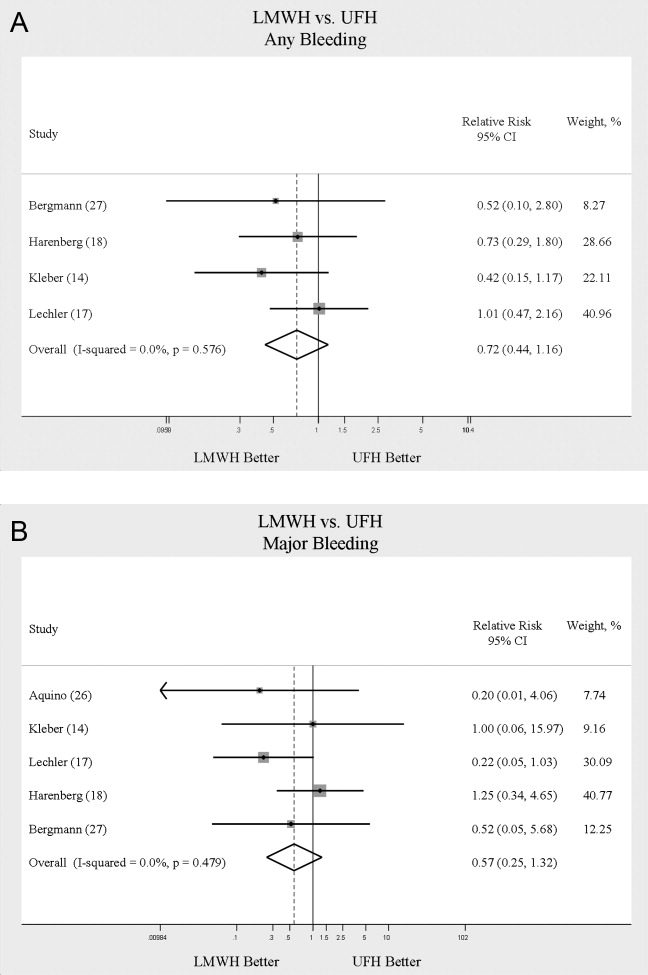
When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).
Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Copyright © 2009 Society of Hospital Medicine
Jumpstarting Hospital Medicine Research
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Copyright © 2008 Society of Hospital Medicine
Contributors to Patient Care Mistakes
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).
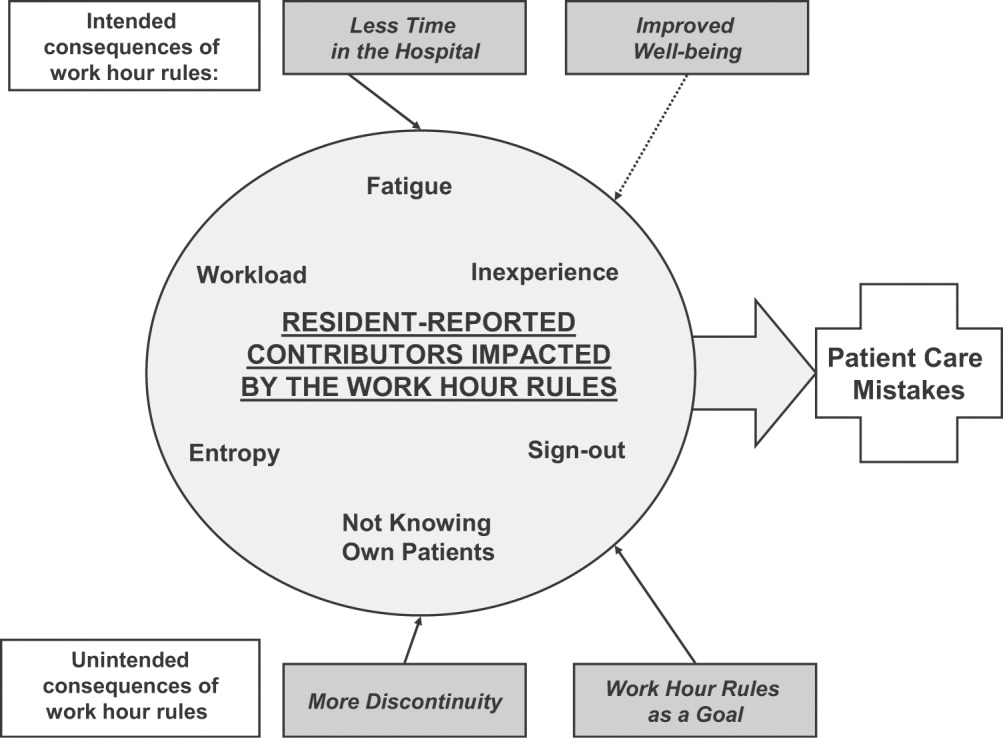
The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Copyright © 2008 Society of Hospital Medicine
Effect of Educational Intervention on Intern Confidence
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).
Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).
DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Copyright © 2006 Society of Hospital Medicine