User login
Adult ADHD: A sensible approach to diagnosis and treatment
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
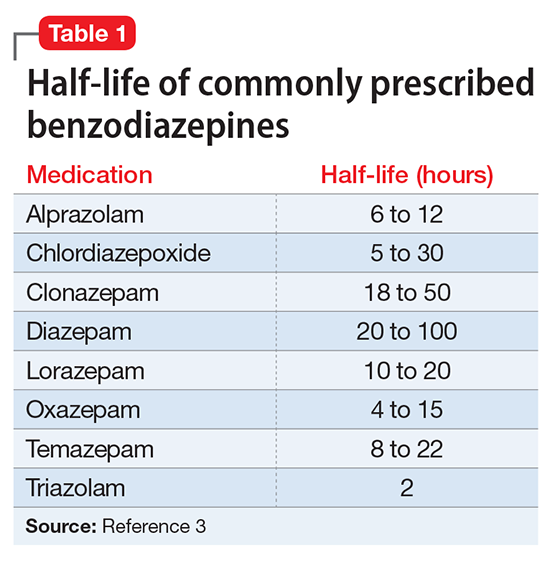
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
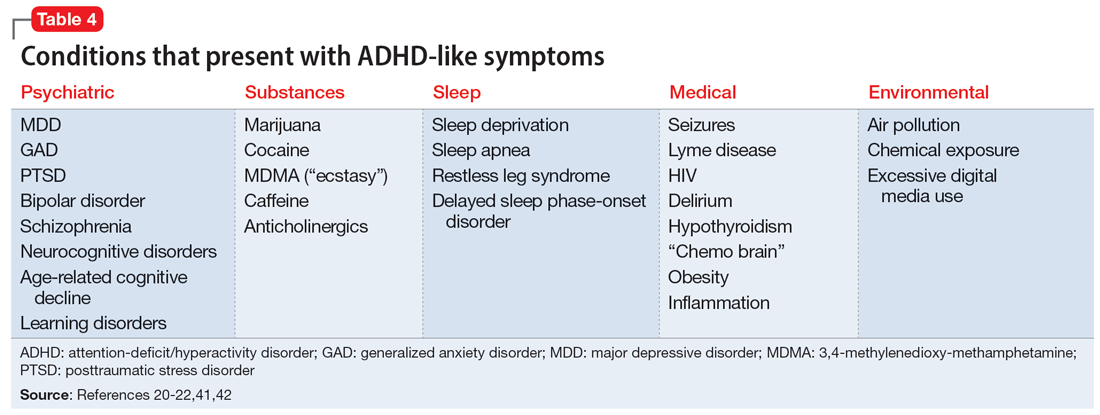
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
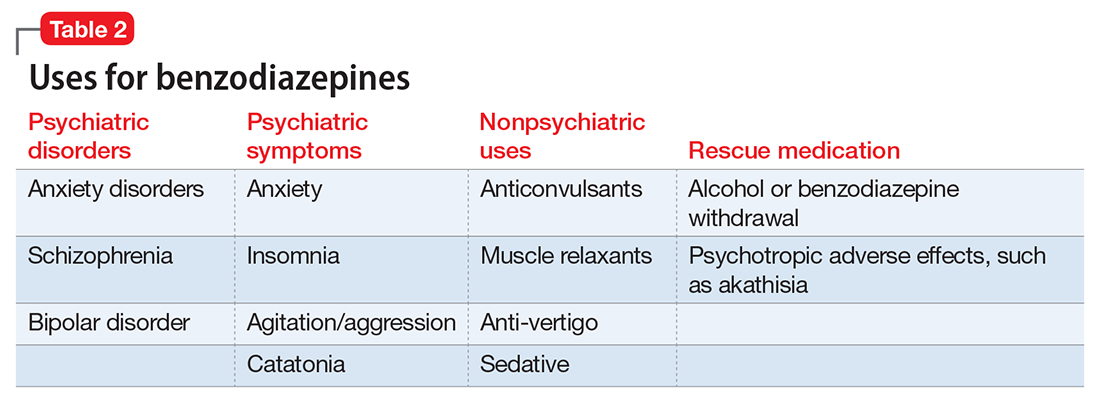
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33
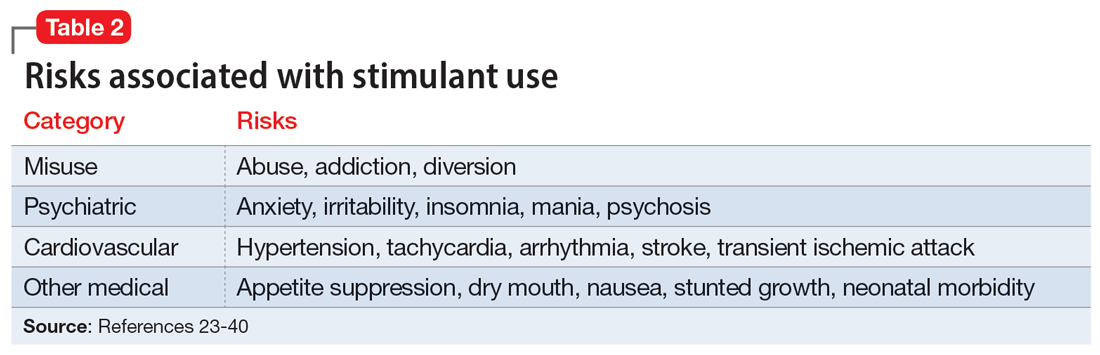
The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
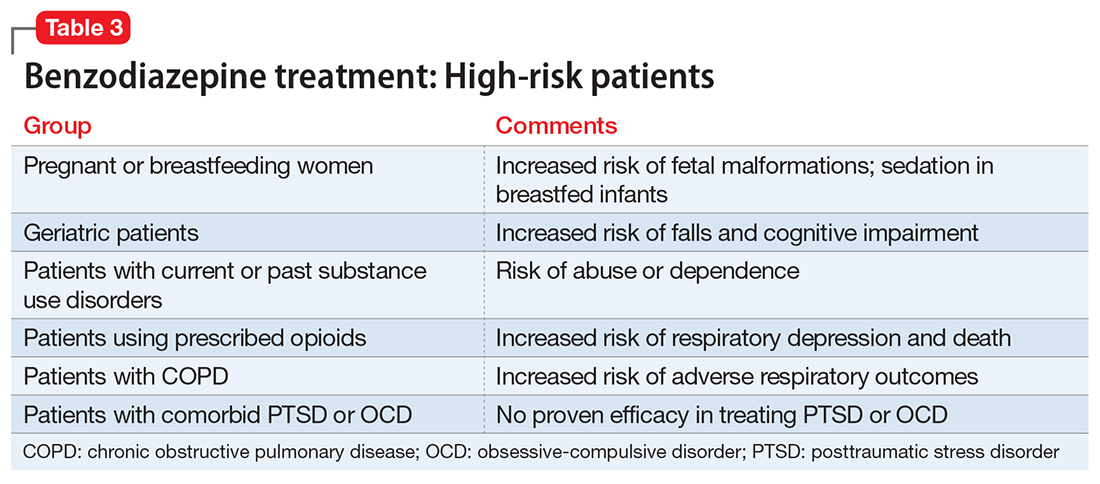
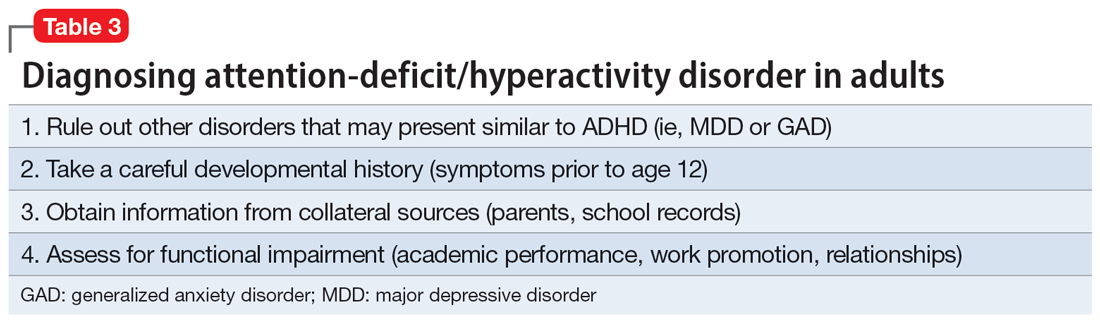
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.
Continue to: Some of the most common conditions...
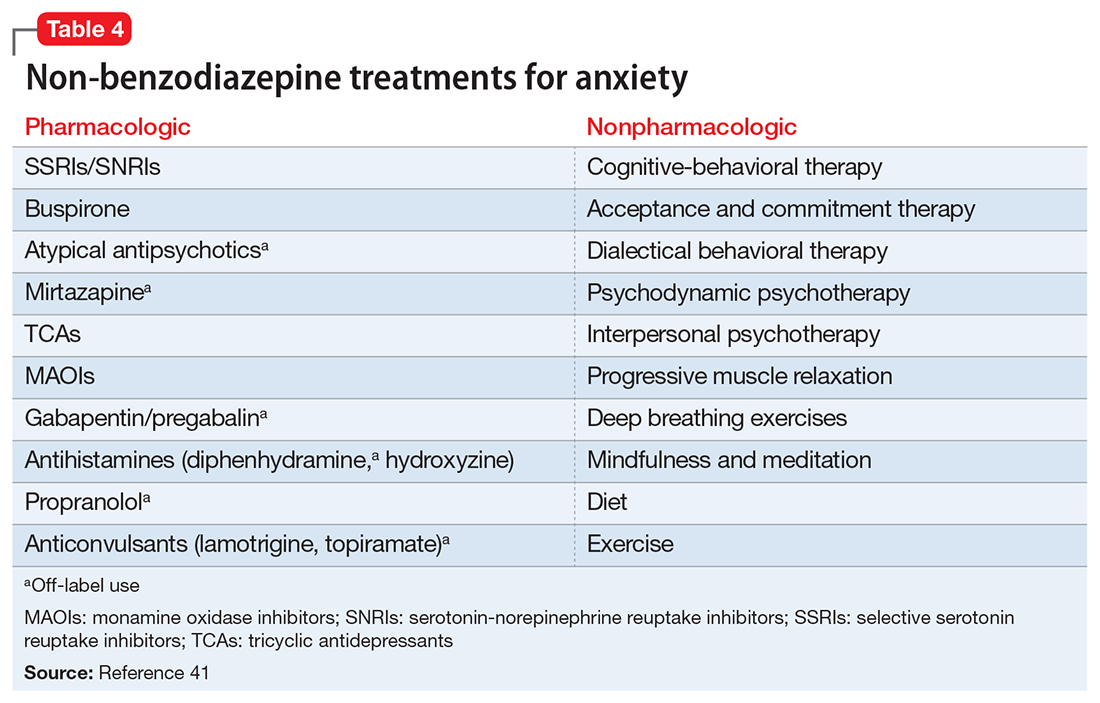
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.
Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48
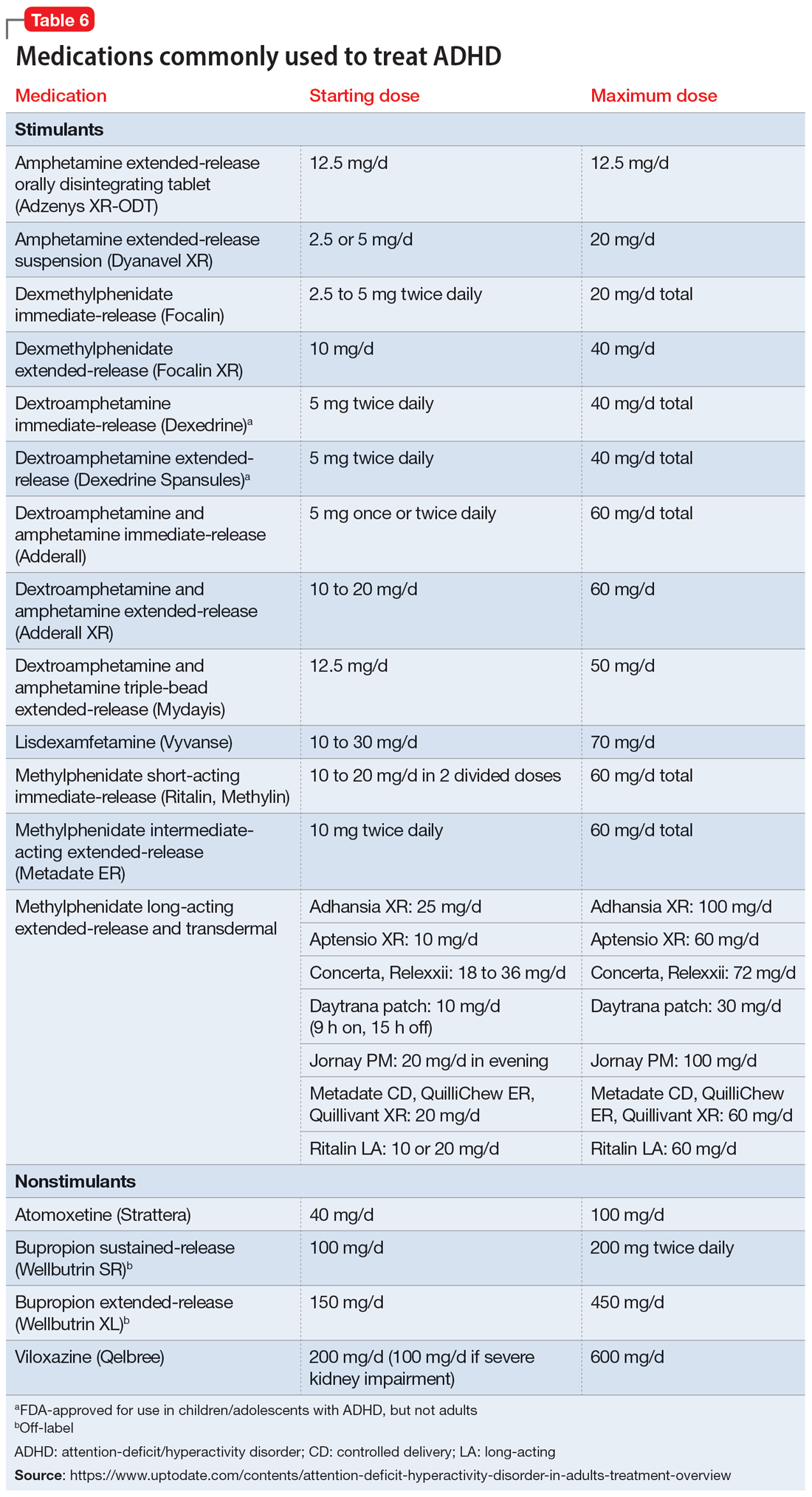
In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Simulating psychoanalysis: A review of Freud’s Bones
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
Contradictions abound in ‘The End of Mental Illness’
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Inaccurate depictions of inpatient psychiatry foster stigma
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
Benzodiazepines: Sensible prescribing in light of the risks
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.