User login
Biceps Tenodesis: A Comparison of Tendon-to-Bone and Tendon-to-Tendon Healing in a Rat Model
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
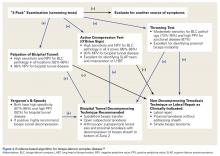
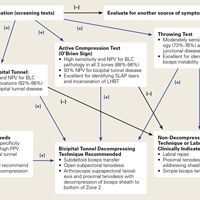
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
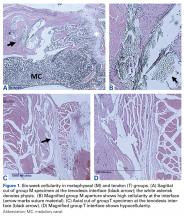
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
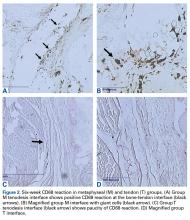
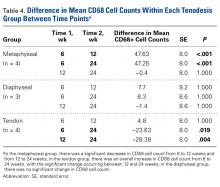
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
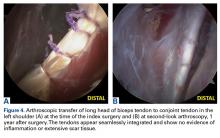
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
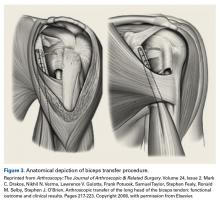
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
Tenotomy, Tenodesis, Transfer: A Review of Treatment Options for Biceps-Labrum Complex Disease
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.