User login
The Role of Dermatology in Identifying and Reporting a Primary Varicella Outbreak
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
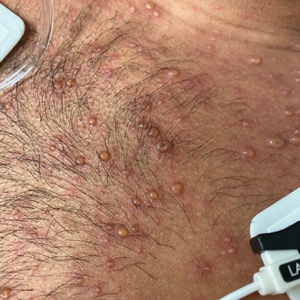
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).
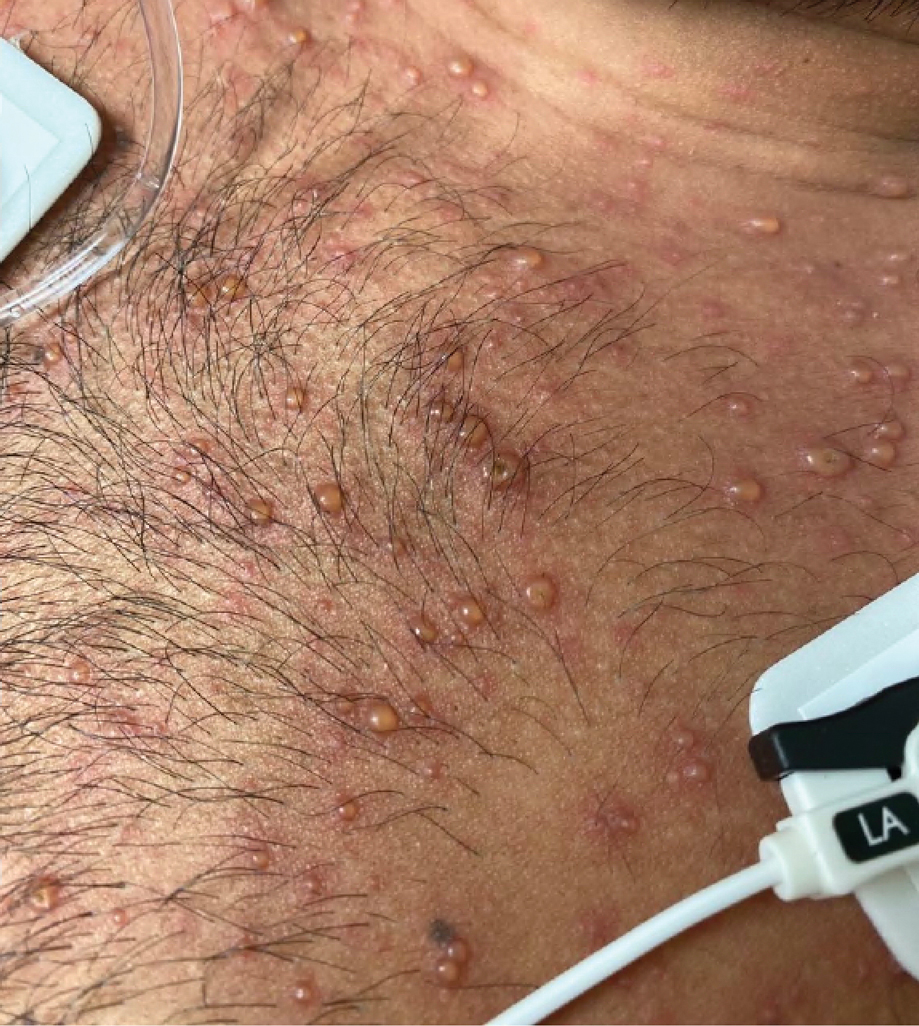
Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).
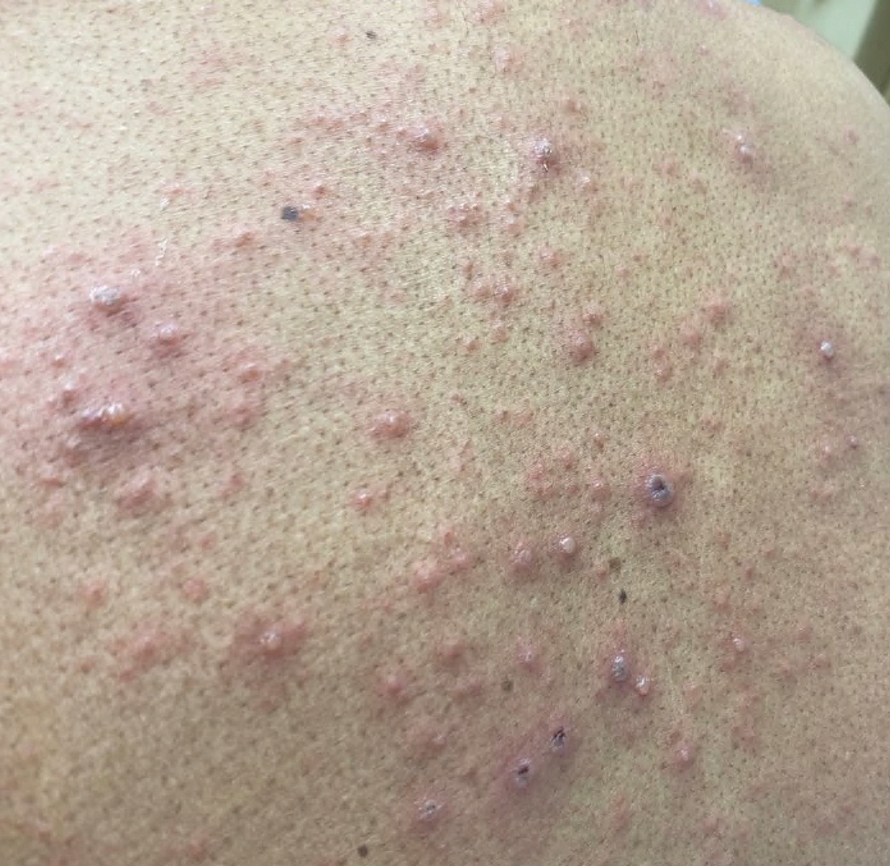
Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
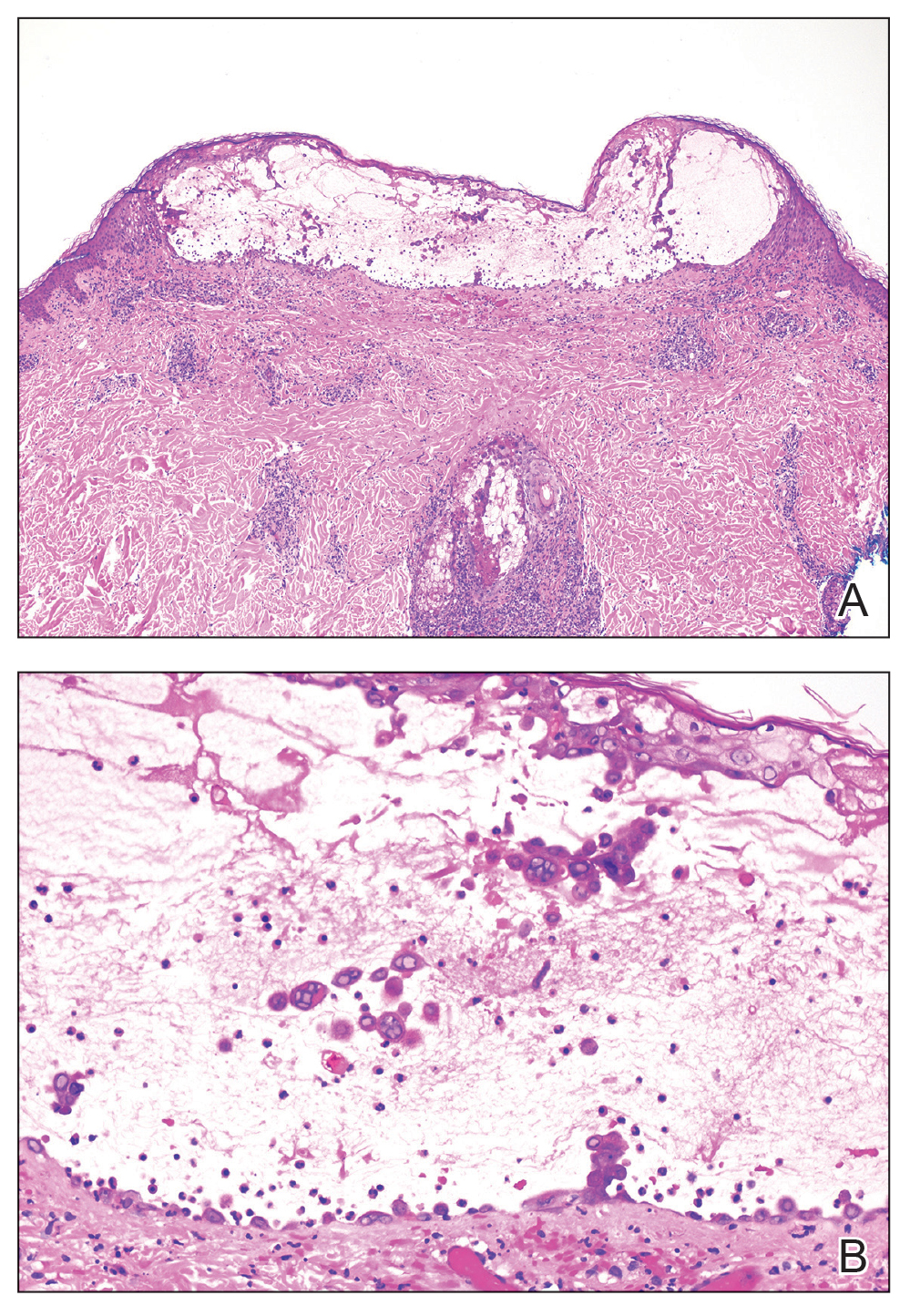
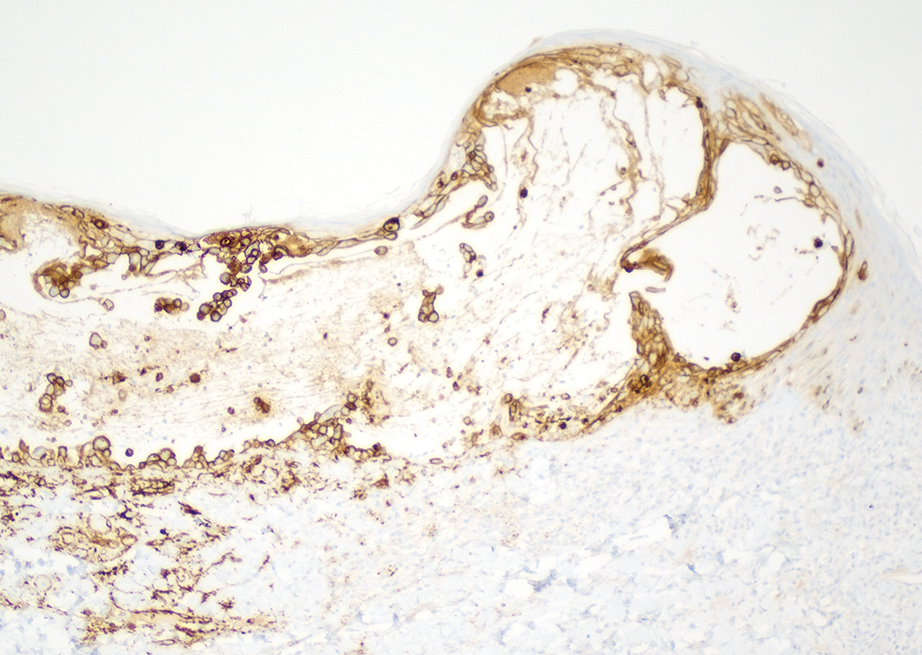
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).
Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).
The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
Practice Points
- Primary varicella is a relatively infrequent occurrence since the introduction of vaccination, creating the need for a reminder on the importance of including it in the differential when clinically appropriate.
- When outbreaks do happen, typically among unvaccinated communities, swift identification via physical examination and histology is imperative to allow infection control teams and public health officials to quickly take action.