User login
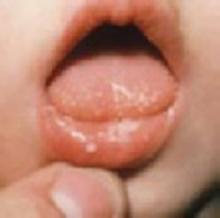
What is the best treatment for oral thrush in healthy infants?
Nystatin oral suspension is a safe first-line therapy; fluconazole is more effective (strength of recommendation [SOR]: B, 1 small randomized controlled trial [RCT]) but has not been approved by the Food and Drug Administration (FDA) for use in immunocompetent infants. Miconazole oral gel is also more effective than nystatin suspension, but is not commercially available in the united states (SOR: B, one small RCT). Gentian violet may be effective, but it stains skin and clothes and is associated with mucosal ulceration (SOR: B, 1 small retrospective cohort study).
Fluconazole isn’t worth the higher cost
Daniel Triezenberg, MD
St. Joseph Regional Medical Center, South Bend, Ind
I reassure parents that oral thrush in infants is rarely a sign of serious illness and recommend nystatin suspension 0.5 cc qid––a smaller dose than reported in this review. Larger doses are more often spit out or swallowed, and at the smaller dose, a 60-mL bottle suppresses the yeast adequately for 2 weeks. My goal is to suppress yeast overgrowth until the infant’s immune system and bacterial flora mature. This review doesn’t convince me that fluconazole, which costs more than nystatin, is worth the added expense. Gentian violet is very messy, and I rarely recommend it. For refractory thrush in breastfed infants, I recommend that the mother apply a topical antifungal to the nipple area.
Evidence summary
Few studies have compared treatment options for oropharyngeal candidiasis in immunocompetent infants. In a survey of 312 health care providers, approximately 75% of the respondents reported treating thrush with oral nystatin, citing fewer side effects and lower cost.1 However, nystatin has proved less effective than either miconazole gel or oral fluconazole.
Nystatin is safe and available, but other options work better
Miconazole vs nystatin. An unblinded RCT assigned 83 immunocompetent infants with culture-positive oral thrush to receive either 25 mg miconazole oral gel (not commercially available in the United States) or nystatin suspension (1 mL of 100,000 IU/mL) qid after meals. The clinical cure rate, defined as absence of plaques by day 12, was significantly higher in the miconazole group (99% for miconazole, 54% for nystatin; P<.0001, number needed to treat [NNT]=2). The eradication rate, confirmed by cultures collected in a blinded manner on the day of clinical cure, was also higher in the miconazole group (55.7% for miconazole, 15.2% for nystatin; P<.0001, NNT=3). In successfully treated patients, infection recurred with similar frequency in both treatment groups within 4 weeks (miconazole, 12.4%; nystatin, 13.0%). Side effects—mostly vomiting and, infrequently, diarrhea—were rare in both groups (miconazole, 4.5%; nystatin 3.5%).2
An earlier, unblinded RCT of 95 infants compared miconazole gel to 2 nystatin oral gels (gel A: 250,000 IU/g with 250,000 IU administered as single dose; gel B: 100,000 IU/g with 50,000 IU administered as single dose). Each medication was given qid over the course of 8 to 14 days. The study confirmed higher clinical cure rates with miconazole gel (85.1% for miconazole vs 42.8% for nystatin gel A [P<.0007, NNT=2] and 48.5% for nystatin gel B [P<.004, NNT=3]).3
Fluconazole vs nystatin. In the only prospective RCT (unblinded) to compare oral suspensions of fluconazole and nystatin, 34 infants were randomized to receive either nystatin (1 mL of 100,000 IU/mL) qid for 10 days or fluconazole (3 mg/kg) once a day for 7 days. Mothers of breastfed infants applied nystatin cream to their nipples twice a day for the duration of the infant’s treatment. The clinical cure rate—defined as absence of oral plaques at the end of therapy (day 10 for the nystatin group, day 7 for the fluconazole group)—was significantly higher in the group treated with fluconazole (100% for fluconazole, 32% for nystatin; P<.0001, NNT=2). The eradication rate was also higher with fluconazole (73.3% for fluconazole, 5.6% for nystatin; P<.0001, NNT=2). The patients treated with fluconazole experienced no side effects.4 Fluconazole has been shown to be effective, safe, and easy to use to treat thrush in immunocompromised children,5 but has not been approved by the FDA for use in healthy infants.
Gentian violet is effective, but messy and irritating
A retrospective cohort study that reviewed 69 cases of oral thrush showed that gentian violet achieved a 75% cure rate in an average of 11 days (compared to 55% in 10 days for nystatin). Both treatments shortened the duration of illness compared with the average of 34 days for untreated children.6 However, gentian violet can stain skin and clothes, and case studies have shown an association with ulceration of the buccal mucosa.7
Recommendations
A thorough literature search through the Cochrane Database Systematic Reviews, Agency for Healthcare Research and Quality, National Guideline Clearinghouse, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on treating oropharyngeal candidiasis in infants. Neither the American Academy of Pediatrics nor the Infectious Diseases Society of America has issued applicable practice guidelines.
1. Brent NB. Thrush in the breastfeeding dyad: results of a survey on diagnosis and treatment. Clin Pediatr. 2001;40:503-506.
2. Hoppe JE. Treatment of oropharyngeal candidiasis in immunocompetent infants: a randomized multicenter study of miconazole gel vs. nystatin suspension. The Antifungals Study Group. Pediatr Infect Dis J. 1997;16:288-293.
3. Hoppe JE, Hahn H. Randomized comparison of two nystatin oral gels with miconazole oral gel for treatment of oral thrush in infants. Antimycotics Study Group. Infection. 1996;24:136-139.
4. Goins RA, Ascher D, Waecker N, et al. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr Infect Dis J. 2002;21:1165-1167.
5. Flynn PM, Cunningham CK, Kerkering T, et al. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J Pediatr. 1995;127:322-328.
6. Kozinn PJ, Taschdjian CL, Dragutsky D, et al. Therapy of oral thrush: a comparative evaluation of gentian violet, mycostatin, and amphotericin B. Monographs on Therapy. 1957;2:16-24.
7. Leung AK. Gentian violet in the treatment of oral candidiasis. Pediatr Infect Dis J. 1988;7:304-305.
Nystatin oral suspension is a safe first-line therapy; fluconazole is more effective (strength of recommendation [SOR]: B, 1 small randomized controlled trial [RCT]) but has not been approved by the Food and Drug Administration (FDA) for use in immunocompetent infants. Miconazole oral gel is also more effective than nystatin suspension, but is not commercially available in the united states (SOR: B, one small RCT). Gentian violet may be effective, but it stains skin and clothes and is associated with mucosal ulceration (SOR: B, 1 small retrospective cohort study).
Fluconazole isn’t worth the higher cost
Daniel Triezenberg, MD
St. Joseph Regional Medical Center, South Bend, Ind
I reassure parents that oral thrush in infants is rarely a sign of serious illness and recommend nystatin suspension 0.5 cc qid––a smaller dose than reported in this review. Larger doses are more often spit out or swallowed, and at the smaller dose, a 60-mL bottle suppresses the yeast adequately for 2 weeks. My goal is to suppress yeast overgrowth until the infant’s immune system and bacterial flora mature. This review doesn’t convince me that fluconazole, which costs more than nystatin, is worth the added expense. Gentian violet is very messy, and I rarely recommend it. For refractory thrush in breastfed infants, I recommend that the mother apply a topical antifungal to the nipple area.
Evidence summary
Few studies have compared treatment options for oropharyngeal candidiasis in immunocompetent infants. In a survey of 312 health care providers, approximately 75% of the respondents reported treating thrush with oral nystatin, citing fewer side effects and lower cost.1 However, nystatin has proved less effective than either miconazole gel or oral fluconazole.
Nystatin is safe and available, but other options work better
Miconazole vs nystatin. An unblinded RCT assigned 83 immunocompetent infants with culture-positive oral thrush to receive either 25 mg miconazole oral gel (not commercially available in the United States) or nystatin suspension (1 mL of 100,000 IU/mL) qid after meals. The clinical cure rate, defined as absence of plaques by day 12, was significantly higher in the miconazole group (99% for miconazole, 54% for nystatin; P<.0001, number needed to treat [NNT]=2). The eradication rate, confirmed by cultures collected in a blinded manner on the day of clinical cure, was also higher in the miconazole group (55.7% for miconazole, 15.2% for nystatin; P<.0001, NNT=3). In successfully treated patients, infection recurred with similar frequency in both treatment groups within 4 weeks (miconazole, 12.4%; nystatin, 13.0%). Side effects—mostly vomiting and, infrequently, diarrhea—were rare in both groups (miconazole, 4.5%; nystatin 3.5%).2
An earlier, unblinded RCT of 95 infants compared miconazole gel to 2 nystatin oral gels (gel A: 250,000 IU/g with 250,000 IU administered as single dose; gel B: 100,000 IU/g with 50,000 IU administered as single dose). Each medication was given qid over the course of 8 to 14 days. The study confirmed higher clinical cure rates with miconazole gel (85.1% for miconazole vs 42.8% for nystatin gel A [P<.0007, NNT=2] and 48.5% for nystatin gel B [P<.004, NNT=3]).3
Fluconazole vs nystatin. In the only prospective RCT (unblinded) to compare oral suspensions of fluconazole and nystatin, 34 infants were randomized to receive either nystatin (1 mL of 100,000 IU/mL) qid for 10 days or fluconazole (3 mg/kg) once a day for 7 days. Mothers of breastfed infants applied nystatin cream to their nipples twice a day for the duration of the infant’s treatment. The clinical cure rate—defined as absence of oral plaques at the end of therapy (day 10 for the nystatin group, day 7 for the fluconazole group)—was significantly higher in the group treated with fluconazole (100% for fluconazole, 32% for nystatin; P<.0001, NNT=2). The eradication rate was also higher with fluconazole (73.3% for fluconazole, 5.6% for nystatin; P<.0001, NNT=2). The patients treated with fluconazole experienced no side effects.4 Fluconazole has been shown to be effective, safe, and easy to use to treat thrush in immunocompromised children,5 but has not been approved by the FDA for use in healthy infants.
Gentian violet is effective, but messy and irritating
A retrospective cohort study that reviewed 69 cases of oral thrush showed that gentian violet achieved a 75% cure rate in an average of 11 days (compared to 55% in 10 days for nystatin). Both treatments shortened the duration of illness compared with the average of 34 days for untreated children.6 However, gentian violet can stain skin and clothes, and case studies have shown an association with ulceration of the buccal mucosa.7
Recommendations
A thorough literature search through the Cochrane Database Systematic Reviews, Agency for Healthcare Research and Quality, National Guideline Clearinghouse, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on treating oropharyngeal candidiasis in infants. Neither the American Academy of Pediatrics nor the Infectious Diseases Society of America has issued applicable practice guidelines.
Nystatin oral suspension is a safe first-line therapy; fluconazole is more effective (strength of recommendation [SOR]: B, 1 small randomized controlled trial [RCT]) but has not been approved by the Food and Drug Administration (FDA) for use in immunocompetent infants. Miconazole oral gel is also more effective than nystatin suspension, but is not commercially available in the united states (SOR: B, one small RCT). Gentian violet may be effective, but it stains skin and clothes and is associated with mucosal ulceration (SOR: B, 1 small retrospective cohort study).
Fluconazole isn’t worth the higher cost
Daniel Triezenberg, MD
St. Joseph Regional Medical Center, South Bend, Ind
I reassure parents that oral thrush in infants is rarely a sign of serious illness and recommend nystatin suspension 0.5 cc qid––a smaller dose than reported in this review. Larger doses are more often spit out or swallowed, and at the smaller dose, a 60-mL bottle suppresses the yeast adequately for 2 weeks. My goal is to suppress yeast overgrowth until the infant’s immune system and bacterial flora mature. This review doesn’t convince me that fluconazole, which costs more than nystatin, is worth the added expense. Gentian violet is very messy, and I rarely recommend it. For refractory thrush in breastfed infants, I recommend that the mother apply a topical antifungal to the nipple area.
Evidence summary
Few studies have compared treatment options for oropharyngeal candidiasis in immunocompetent infants. In a survey of 312 health care providers, approximately 75% of the respondents reported treating thrush with oral nystatin, citing fewer side effects and lower cost.1 However, nystatin has proved less effective than either miconazole gel or oral fluconazole.
Nystatin is safe and available, but other options work better
Miconazole vs nystatin. An unblinded RCT assigned 83 immunocompetent infants with culture-positive oral thrush to receive either 25 mg miconazole oral gel (not commercially available in the United States) or nystatin suspension (1 mL of 100,000 IU/mL) qid after meals. The clinical cure rate, defined as absence of plaques by day 12, was significantly higher in the miconazole group (99% for miconazole, 54% for nystatin; P<.0001, number needed to treat [NNT]=2). The eradication rate, confirmed by cultures collected in a blinded manner on the day of clinical cure, was also higher in the miconazole group (55.7% for miconazole, 15.2% for nystatin; P<.0001, NNT=3). In successfully treated patients, infection recurred with similar frequency in both treatment groups within 4 weeks (miconazole, 12.4%; nystatin, 13.0%). Side effects—mostly vomiting and, infrequently, diarrhea—were rare in both groups (miconazole, 4.5%; nystatin 3.5%).2
An earlier, unblinded RCT of 95 infants compared miconazole gel to 2 nystatin oral gels (gel A: 250,000 IU/g with 250,000 IU administered as single dose; gel B: 100,000 IU/g with 50,000 IU administered as single dose). Each medication was given qid over the course of 8 to 14 days. The study confirmed higher clinical cure rates with miconazole gel (85.1% for miconazole vs 42.8% for nystatin gel A [P<.0007, NNT=2] and 48.5% for nystatin gel B [P<.004, NNT=3]).3
Fluconazole vs nystatin. In the only prospective RCT (unblinded) to compare oral suspensions of fluconazole and nystatin, 34 infants were randomized to receive either nystatin (1 mL of 100,000 IU/mL) qid for 10 days or fluconazole (3 mg/kg) once a day for 7 days. Mothers of breastfed infants applied nystatin cream to their nipples twice a day for the duration of the infant’s treatment. The clinical cure rate—defined as absence of oral plaques at the end of therapy (day 10 for the nystatin group, day 7 for the fluconazole group)—was significantly higher in the group treated with fluconazole (100% for fluconazole, 32% for nystatin; P<.0001, NNT=2). The eradication rate was also higher with fluconazole (73.3% for fluconazole, 5.6% for nystatin; P<.0001, NNT=2). The patients treated with fluconazole experienced no side effects.4 Fluconazole has been shown to be effective, safe, and easy to use to treat thrush in immunocompromised children,5 but has not been approved by the FDA for use in healthy infants.
Gentian violet is effective, but messy and irritating
A retrospective cohort study that reviewed 69 cases of oral thrush showed that gentian violet achieved a 75% cure rate in an average of 11 days (compared to 55% in 10 days for nystatin). Both treatments shortened the duration of illness compared with the average of 34 days for untreated children.6 However, gentian violet can stain skin and clothes, and case studies have shown an association with ulceration of the buccal mucosa.7
Recommendations
A thorough literature search through the Cochrane Database Systematic Reviews, Agency for Healthcare Research and Quality, National Guideline Clearinghouse, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on treating oropharyngeal candidiasis in infants. Neither the American Academy of Pediatrics nor the Infectious Diseases Society of America has issued applicable practice guidelines.
1. Brent NB. Thrush in the breastfeeding dyad: results of a survey on diagnosis and treatment. Clin Pediatr. 2001;40:503-506.
2. Hoppe JE. Treatment of oropharyngeal candidiasis in immunocompetent infants: a randomized multicenter study of miconazole gel vs. nystatin suspension. The Antifungals Study Group. Pediatr Infect Dis J. 1997;16:288-293.
3. Hoppe JE, Hahn H. Randomized comparison of two nystatin oral gels with miconazole oral gel for treatment of oral thrush in infants. Antimycotics Study Group. Infection. 1996;24:136-139.
4. Goins RA, Ascher D, Waecker N, et al. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr Infect Dis J. 2002;21:1165-1167.
5. Flynn PM, Cunningham CK, Kerkering T, et al. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J Pediatr. 1995;127:322-328.
6. Kozinn PJ, Taschdjian CL, Dragutsky D, et al. Therapy of oral thrush: a comparative evaluation of gentian violet, mycostatin, and amphotericin B. Monographs on Therapy. 1957;2:16-24.
7. Leung AK. Gentian violet in the treatment of oral candidiasis. Pediatr Infect Dis J. 1988;7:304-305.
1. Brent NB. Thrush in the breastfeeding dyad: results of a survey on diagnosis and treatment. Clin Pediatr. 2001;40:503-506.
2. Hoppe JE. Treatment of oropharyngeal candidiasis in immunocompetent infants: a randomized multicenter study of miconazole gel vs. nystatin suspension. The Antifungals Study Group. Pediatr Infect Dis J. 1997;16:288-293.
3. Hoppe JE, Hahn H. Randomized comparison of two nystatin oral gels with miconazole oral gel for treatment of oral thrush in infants. Antimycotics Study Group. Infection. 1996;24:136-139.
4. Goins RA, Ascher D, Waecker N, et al. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr Infect Dis J. 2002;21:1165-1167.
5. Flynn PM, Cunningham CK, Kerkering T, et al. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J Pediatr. 1995;127:322-328.
6. Kozinn PJ, Taschdjian CL, Dragutsky D, et al. Therapy of oral thrush: a comparative evaluation of gentian violet, mycostatin, and amphotericin B. Monographs on Therapy. 1957;2:16-24.
7. Leung AK. Gentian violet in the treatment of oral candidiasis. Pediatr Infect Dis J. 1988;7:304-305.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best diagnostic evaluation of night sweats?
There is no single best evidence-based approach to the diagnostic evaluation of night sweats, given the limited number of studies on the subject. A detailed history, however, does appear to be the most important initial diagnostic tool (strength of recommendation [SOR]: C, based on usual practice and clinical opinion).
No clinical trials have directly studied symptomatic relief of night sweats alone. Among menopausal women with hot flashes associated with night sweats, oral hormone therapy is highly effective in reducing their frequency (SOR: A, based on a Cochrane review with a clear recommendation). Antireflux therapy may also be effective (SOR: B, based on a cohort study). Therapy aimed at decreasing perspiration has also been suggested (SOR: C, based on clinical opinion.)
Night sweats are an increasingly common complaint
Lisa Johnson, MD
Providence Health Care Systems, University of Washington, Seattle
Complaints of night sweats among my menopausal patients have become very common with the declining use of hormone replacement therapy. Both women and their bed partners are affected, and sleep deprivation is a significant side effect, so the problem must be taken seriously.
Though venlafaxine can cause night sweats, it is also a reasonable treatment strategy for menopause-related night sweats. Gabapentin may hold promise for hormonal symptoms if reflux is not the issue. Other sinister causes of night sweats are uncommon, but are always in the back of my mind when the issue is raised, so the history and review of systems help focus the work-up. The pretest probability of unusual diagnoses guides specific laboratory testing.
Evidence summary
Night sweats are a common complaint in the ambulatory primary care setting: Of 2267 patients in 1 cross-sectional study, 41% reported night sweats, defined as “sweating at night even when it isn’t excessively hot in your bedroom” within the previous month.1 Because the peak prevalence in both men and women occurred in the group ages 41 to 55 years, there was concern that menopausal hot flashes were a confounding factor, at least for women. In a subsequent study of 795 patients older than 64 years, 10% still reported being bothered by night sweats.2
The more common causes are not widely studied
Few studies look at the causes of night sweats. Although they have been associated with tuberculosis, lymphoma, and HIV infection, these are not common causes of night sweats in outpatient care.
In the only study that specifically addressed the causes of night sweats in an ambulatory population, Reynolds3 interviewed 200 consecutive patients, 70% from a primary care practice and 30% from a gastroenterology practice. Of the 81 patients who reported having an episode of night sweats at least once a week, esophageal reflux and menopause were the most frequent causes.
Several authors agree that certain medications are frequently associated with night sweats, although the exact incidence is unknown due to a lack of published epidemiologic data.4-6 Antidepressants and antipyretics are among the more commonly cited offenders (TABLE 1).4
TABLE 1
Medications that may cause sweating or flushing
| ANTIDEPRESSANTS |
| Bupropion (Wellbutrin) |
| SSRIs |
| Tricyclic antidepressants |
| Venlafaxine (Effexor) |
| ANTIMIGRAINE DRUGS |
| Naratriptan (Amerge) |
| Rizatriptan (Maxalt) |
| Sumatriptan (Imitrex) |
| Zolmitriptan (Zomig) |
| ANTIPYRETICS |
| Acetaminophen |
| Aspirin |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) |
| CHOLINERGIC AGONISTS |
| Bethanechol (urecholine) |
| Pilocarpine |
| GNRH AGONISTS |
| Gonadorelin |
| Goserelin (Zoladex) |
| Histrelin (Vantas) |
| Leuprolide (Lupron) |
| Nafarelin (Synarel) |
| HYPOGLYCEMIC AGENTS |
| Insulin |
| Sulfonylureas |
| SYMPATHOMIMETIC AGENTS |
| Beta-agonists |
| Phenylephrine (sudafed) |
| OTHER AGENTS |
| Alcohol |
| Beta-blockers |
| Bromocriptine (Parodel) |
| Calcium channel blockers |
| Clozapine (Clozaril) |
| Cyclosporine |
| Hydralazine (Hydra-Zide) |
| Niacin |
| Nitroglycerin |
| Omeprazole (Prilosec) |
| Opioids |
| sildenafil (Viagra) |
| Tamoxifen (Nolvadex) |
| Theophylline |
| Tramadol (Ultram, Ultracet) |
| Source: UpToDate.4 |
Finding the right diagnosis requires thorough history & exam
With such a long differential diagnosis (TABLE 2),4-6 night sweats should initially be evaluated with a thorough history and physical examination (according to a consensus opinion of various authors). If these don’t elicit possible causes, the appropriate next step in the work-up can vary. Some authors recommend multiple laboratory and imaging studies, while others advise against any routine tests. None of these approaches is evidence-based.
One reasonable algorithm recommends an initial work-up including a complete blood count, thyroid-stimulating hormone (TSH) and erythrocyte sedimentation rate (ESR) level, a purified protein derivative (PPD) and HIV test, and a chest x-ray.5 If the results are unrevealing, a trial of antireflux medication is recommended. If the patient does not improve, consider a diary of nocturnal temperatures to help discern the presence or absence of febrile pulses and further evaluate for suspected endocarditis or lymphoma.
TABLE 2
Differential diagnosis for night sweats
| ENDOCRINE |
| Carcinoid syndrome |
| Diabetes insipidus |
| Hyperthyroidism |
| Hypoglycemia |
| Pheochromocytoma |
| Post-orchiectomy |
| INFECTIONS |
| Coccidioidomycosis |
| Endocarditis |
| Histoplasmosis |
| Human immunodeficiency virus |
| Infectious mononucleosis |
| Lung abscess |
| Mycobacterium avium complex |
| Osteomyelitis |
| Tuberculosis |
| MALIGNANCY |
| Leukemia |
| Lymphoma |
| Prostate cancer |
| Renal cell carcinoma |
| Other neoplasms |
| NEUROLOGIC DISORDERS |
| Autonomic dysreflexia |
| Autonomic neuropathy |
| Stroke |
| SUBSTANCE WITHDRAWAL |
| Alcohol |
| Cocaine |
| Opioids |
| MISCELLANEOUS |
| Chronic fatigue syndrome |
| Gastroesophageal reflux disease |
| Menopause |
| Obstructive sleep disorder |
| Panic disorder |
| Pregnancy |
| Prinzmetal’s angina |
| Takayasu’s arteritis |
| Temporal arteritis |
| Source: UpToDate;4 viera et al, Am Fam Physician 2003;5 Chambliss, Arch Fam Med 1999.6 |
Evidence is scant for symptom relief
Very few clinical trials have directly studied symptomatic relief of night sweats. A large Cochrane meta-analysis found that oral hormone therapy—estrogens alone or estrogens with progesterone—reduced the frequency of night sweats associated with hot flashes among menopausal women by 75% when compared with placebo alone.7 Neither primrose oil nor foot reflexology proved effective.8
A cohort study found that 80% of the patients with frequent night sweats responded to antireflux therapy.3 One author suggests using therapies aimed at relieving hyperhydrosis.6 These include local treatment with aluminum chloride hexahydrate (Drysol), antiperspirants, scopolamine, or phenoxybenzamine hydrochloride (Dibenzyline).
Recommendations from others
A thorough literature search through Cochrane Database Systematic Reviews, AHRQ, National Guideline Clearing-house, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on the evaluation or treatment of night sweats.
1. Mold JW, Mathew MK, Belgore S, Dehaven M. Prevalence of night sweats in primary care patients: an OKPRN and TAFP-Net collaborative study. J Fam Pract 2002;51:452-456.
2. Mold JW, Roberts M, Aboshady HM. Prevalence and predictors of night sweats, day sweats, and hot flashes in older primary care patients: an OKPRN study. Ann Fam Med 2004;2:391-397.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? J Clin Gastroenenterol 1989;11:590-591.
4. Smetana GW. Approach to the patient with night sweats. UpToDate [database online]. Updated October 3, 2006. Available at: www.uptodate.com.
5. Viera AJ, Bond MM, Yates SW. Diagnosing night sweats. Am Fam Physician 2003;67:1019-1024.
6. Chambliss ML. What is the appropriate diagnostic approach for patients who complain of night sweats? Arch Fam Med 1999;8:168-169.
7. MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Datab Syst Rev 2004;CD002978.-
8. Williamson J, White A, Hart A, Ernst E. Randomised controlled trial of reflexology for menopausal symptoms. BJOG 2002;109:1050-1055.
There is no single best evidence-based approach to the diagnostic evaluation of night sweats, given the limited number of studies on the subject. A detailed history, however, does appear to be the most important initial diagnostic tool (strength of recommendation [SOR]: C, based on usual practice and clinical opinion).
No clinical trials have directly studied symptomatic relief of night sweats alone. Among menopausal women with hot flashes associated with night sweats, oral hormone therapy is highly effective in reducing their frequency (SOR: A, based on a Cochrane review with a clear recommendation). Antireflux therapy may also be effective (SOR: B, based on a cohort study). Therapy aimed at decreasing perspiration has also been suggested (SOR: C, based on clinical opinion.)
Night sweats are an increasingly common complaint
Lisa Johnson, MD
Providence Health Care Systems, University of Washington, Seattle
Complaints of night sweats among my menopausal patients have become very common with the declining use of hormone replacement therapy. Both women and their bed partners are affected, and sleep deprivation is a significant side effect, so the problem must be taken seriously.
Though venlafaxine can cause night sweats, it is also a reasonable treatment strategy for menopause-related night sweats. Gabapentin may hold promise for hormonal symptoms if reflux is not the issue. Other sinister causes of night sweats are uncommon, but are always in the back of my mind when the issue is raised, so the history and review of systems help focus the work-up. The pretest probability of unusual diagnoses guides specific laboratory testing.
Evidence summary
Night sweats are a common complaint in the ambulatory primary care setting: Of 2267 patients in 1 cross-sectional study, 41% reported night sweats, defined as “sweating at night even when it isn’t excessively hot in your bedroom” within the previous month.1 Because the peak prevalence in both men and women occurred in the group ages 41 to 55 years, there was concern that menopausal hot flashes were a confounding factor, at least for women. In a subsequent study of 795 patients older than 64 years, 10% still reported being bothered by night sweats.2
The more common causes are not widely studied
Few studies look at the causes of night sweats. Although they have been associated with tuberculosis, lymphoma, and HIV infection, these are not common causes of night sweats in outpatient care.
In the only study that specifically addressed the causes of night sweats in an ambulatory population, Reynolds3 interviewed 200 consecutive patients, 70% from a primary care practice and 30% from a gastroenterology practice. Of the 81 patients who reported having an episode of night sweats at least once a week, esophageal reflux and menopause were the most frequent causes.
Several authors agree that certain medications are frequently associated with night sweats, although the exact incidence is unknown due to a lack of published epidemiologic data.4-6 Antidepressants and antipyretics are among the more commonly cited offenders (TABLE 1).4
TABLE 1
Medications that may cause sweating or flushing
| ANTIDEPRESSANTS |
| Bupropion (Wellbutrin) |
| SSRIs |
| Tricyclic antidepressants |
| Venlafaxine (Effexor) |
| ANTIMIGRAINE DRUGS |
| Naratriptan (Amerge) |
| Rizatriptan (Maxalt) |
| Sumatriptan (Imitrex) |
| Zolmitriptan (Zomig) |
| ANTIPYRETICS |
| Acetaminophen |
| Aspirin |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) |
| CHOLINERGIC AGONISTS |
| Bethanechol (urecholine) |
| Pilocarpine |
| GNRH AGONISTS |
| Gonadorelin |
| Goserelin (Zoladex) |
| Histrelin (Vantas) |
| Leuprolide (Lupron) |
| Nafarelin (Synarel) |
| HYPOGLYCEMIC AGENTS |
| Insulin |
| Sulfonylureas |
| SYMPATHOMIMETIC AGENTS |
| Beta-agonists |
| Phenylephrine (sudafed) |
| OTHER AGENTS |
| Alcohol |
| Beta-blockers |
| Bromocriptine (Parodel) |
| Calcium channel blockers |
| Clozapine (Clozaril) |
| Cyclosporine |
| Hydralazine (Hydra-Zide) |
| Niacin |
| Nitroglycerin |
| Omeprazole (Prilosec) |
| Opioids |
| sildenafil (Viagra) |
| Tamoxifen (Nolvadex) |
| Theophylline |
| Tramadol (Ultram, Ultracet) |
| Source: UpToDate.4 |
Finding the right diagnosis requires thorough history & exam
With such a long differential diagnosis (TABLE 2),4-6 night sweats should initially be evaluated with a thorough history and physical examination (according to a consensus opinion of various authors). If these don’t elicit possible causes, the appropriate next step in the work-up can vary. Some authors recommend multiple laboratory and imaging studies, while others advise against any routine tests. None of these approaches is evidence-based.
One reasonable algorithm recommends an initial work-up including a complete blood count, thyroid-stimulating hormone (TSH) and erythrocyte sedimentation rate (ESR) level, a purified protein derivative (PPD) and HIV test, and a chest x-ray.5 If the results are unrevealing, a trial of antireflux medication is recommended. If the patient does not improve, consider a diary of nocturnal temperatures to help discern the presence or absence of febrile pulses and further evaluate for suspected endocarditis or lymphoma.
TABLE 2
Differential diagnosis for night sweats
| ENDOCRINE |
| Carcinoid syndrome |
| Diabetes insipidus |
| Hyperthyroidism |
| Hypoglycemia |
| Pheochromocytoma |
| Post-orchiectomy |
| INFECTIONS |
| Coccidioidomycosis |
| Endocarditis |
| Histoplasmosis |
| Human immunodeficiency virus |
| Infectious mononucleosis |
| Lung abscess |
| Mycobacterium avium complex |
| Osteomyelitis |
| Tuberculosis |
| MALIGNANCY |
| Leukemia |
| Lymphoma |
| Prostate cancer |
| Renal cell carcinoma |
| Other neoplasms |
| NEUROLOGIC DISORDERS |
| Autonomic dysreflexia |
| Autonomic neuropathy |
| Stroke |
| SUBSTANCE WITHDRAWAL |
| Alcohol |
| Cocaine |
| Opioids |
| MISCELLANEOUS |
| Chronic fatigue syndrome |
| Gastroesophageal reflux disease |
| Menopause |
| Obstructive sleep disorder |
| Panic disorder |
| Pregnancy |
| Prinzmetal’s angina |
| Takayasu’s arteritis |
| Temporal arteritis |
| Source: UpToDate;4 viera et al, Am Fam Physician 2003;5 Chambliss, Arch Fam Med 1999.6 |
Evidence is scant for symptom relief
Very few clinical trials have directly studied symptomatic relief of night sweats. A large Cochrane meta-analysis found that oral hormone therapy—estrogens alone or estrogens with progesterone—reduced the frequency of night sweats associated with hot flashes among menopausal women by 75% when compared with placebo alone.7 Neither primrose oil nor foot reflexology proved effective.8
A cohort study found that 80% of the patients with frequent night sweats responded to antireflux therapy.3 One author suggests using therapies aimed at relieving hyperhydrosis.6 These include local treatment with aluminum chloride hexahydrate (Drysol), antiperspirants, scopolamine, or phenoxybenzamine hydrochloride (Dibenzyline).
Recommendations from others
A thorough literature search through Cochrane Database Systematic Reviews, AHRQ, National Guideline Clearing-house, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on the evaluation or treatment of night sweats.
There is no single best evidence-based approach to the diagnostic evaluation of night sweats, given the limited number of studies on the subject. A detailed history, however, does appear to be the most important initial diagnostic tool (strength of recommendation [SOR]: C, based on usual practice and clinical opinion).
No clinical trials have directly studied symptomatic relief of night sweats alone. Among menopausal women with hot flashes associated with night sweats, oral hormone therapy is highly effective in reducing their frequency (SOR: A, based on a Cochrane review with a clear recommendation). Antireflux therapy may also be effective (SOR: B, based on a cohort study). Therapy aimed at decreasing perspiration has also been suggested (SOR: C, based on clinical opinion.)
Night sweats are an increasingly common complaint
Lisa Johnson, MD
Providence Health Care Systems, University of Washington, Seattle
Complaints of night sweats among my menopausal patients have become very common with the declining use of hormone replacement therapy. Both women and their bed partners are affected, and sleep deprivation is a significant side effect, so the problem must be taken seriously.
Though venlafaxine can cause night sweats, it is also a reasonable treatment strategy for menopause-related night sweats. Gabapentin may hold promise for hormonal symptoms if reflux is not the issue. Other sinister causes of night sweats are uncommon, but are always in the back of my mind when the issue is raised, so the history and review of systems help focus the work-up. The pretest probability of unusual diagnoses guides specific laboratory testing.
Evidence summary
Night sweats are a common complaint in the ambulatory primary care setting: Of 2267 patients in 1 cross-sectional study, 41% reported night sweats, defined as “sweating at night even when it isn’t excessively hot in your bedroom” within the previous month.1 Because the peak prevalence in both men and women occurred in the group ages 41 to 55 years, there was concern that menopausal hot flashes were a confounding factor, at least for women. In a subsequent study of 795 patients older than 64 years, 10% still reported being bothered by night sweats.2
The more common causes are not widely studied
Few studies look at the causes of night sweats. Although they have been associated with tuberculosis, lymphoma, and HIV infection, these are not common causes of night sweats in outpatient care.
In the only study that specifically addressed the causes of night sweats in an ambulatory population, Reynolds3 interviewed 200 consecutive patients, 70% from a primary care practice and 30% from a gastroenterology practice. Of the 81 patients who reported having an episode of night sweats at least once a week, esophageal reflux and menopause were the most frequent causes.
Several authors agree that certain medications are frequently associated with night sweats, although the exact incidence is unknown due to a lack of published epidemiologic data.4-6 Antidepressants and antipyretics are among the more commonly cited offenders (TABLE 1).4
TABLE 1
Medications that may cause sweating or flushing
| ANTIDEPRESSANTS |
| Bupropion (Wellbutrin) |
| SSRIs |
| Tricyclic antidepressants |
| Venlafaxine (Effexor) |
| ANTIMIGRAINE DRUGS |
| Naratriptan (Amerge) |
| Rizatriptan (Maxalt) |
| Sumatriptan (Imitrex) |
| Zolmitriptan (Zomig) |
| ANTIPYRETICS |
| Acetaminophen |
| Aspirin |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) |
| CHOLINERGIC AGONISTS |
| Bethanechol (urecholine) |
| Pilocarpine |
| GNRH AGONISTS |
| Gonadorelin |
| Goserelin (Zoladex) |
| Histrelin (Vantas) |
| Leuprolide (Lupron) |
| Nafarelin (Synarel) |
| HYPOGLYCEMIC AGENTS |
| Insulin |
| Sulfonylureas |
| SYMPATHOMIMETIC AGENTS |
| Beta-agonists |
| Phenylephrine (sudafed) |
| OTHER AGENTS |
| Alcohol |
| Beta-blockers |
| Bromocriptine (Parodel) |
| Calcium channel blockers |
| Clozapine (Clozaril) |
| Cyclosporine |
| Hydralazine (Hydra-Zide) |
| Niacin |
| Nitroglycerin |
| Omeprazole (Prilosec) |
| Opioids |
| sildenafil (Viagra) |
| Tamoxifen (Nolvadex) |
| Theophylline |
| Tramadol (Ultram, Ultracet) |
| Source: UpToDate.4 |
Finding the right diagnosis requires thorough history & exam
With such a long differential diagnosis (TABLE 2),4-6 night sweats should initially be evaluated with a thorough history and physical examination (according to a consensus opinion of various authors). If these don’t elicit possible causes, the appropriate next step in the work-up can vary. Some authors recommend multiple laboratory and imaging studies, while others advise against any routine tests. None of these approaches is evidence-based.
One reasonable algorithm recommends an initial work-up including a complete blood count, thyroid-stimulating hormone (TSH) and erythrocyte sedimentation rate (ESR) level, a purified protein derivative (PPD) and HIV test, and a chest x-ray.5 If the results are unrevealing, a trial of antireflux medication is recommended. If the patient does not improve, consider a diary of nocturnal temperatures to help discern the presence or absence of febrile pulses and further evaluate for suspected endocarditis or lymphoma.
TABLE 2
Differential diagnosis for night sweats
| ENDOCRINE |
| Carcinoid syndrome |
| Diabetes insipidus |
| Hyperthyroidism |
| Hypoglycemia |
| Pheochromocytoma |
| Post-orchiectomy |
| INFECTIONS |
| Coccidioidomycosis |
| Endocarditis |
| Histoplasmosis |
| Human immunodeficiency virus |
| Infectious mononucleosis |
| Lung abscess |
| Mycobacterium avium complex |
| Osteomyelitis |
| Tuberculosis |
| MALIGNANCY |
| Leukemia |
| Lymphoma |
| Prostate cancer |
| Renal cell carcinoma |
| Other neoplasms |
| NEUROLOGIC DISORDERS |
| Autonomic dysreflexia |
| Autonomic neuropathy |
| Stroke |
| SUBSTANCE WITHDRAWAL |
| Alcohol |
| Cocaine |
| Opioids |
| MISCELLANEOUS |
| Chronic fatigue syndrome |
| Gastroesophageal reflux disease |
| Menopause |
| Obstructive sleep disorder |
| Panic disorder |
| Pregnancy |
| Prinzmetal’s angina |
| Takayasu’s arteritis |
| Temporal arteritis |
| Source: UpToDate;4 viera et al, Am Fam Physician 2003;5 Chambliss, Arch Fam Med 1999.6 |
Evidence is scant for symptom relief
Very few clinical trials have directly studied symptomatic relief of night sweats. A large Cochrane meta-analysis found that oral hormone therapy—estrogens alone or estrogens with progesterone—reduced the frequency of night sweats associated with hot flashes among menopausal women by 75% when compared with placebo alone.7 Neither primrose oil nor foot reflexology proved effective.8
A cohort study found that 80% of the patients with frequent night sweats responded to antireflux therapy.3 One author suggests using therapies aimed at relieving hyperhydrosis.6 These include local treatment with aluminum chloride hexahydrate (Drysol), antiperspirants, scopolamine, or phenoxybenzamine hydrochloride (Dibenzyline).
Recommendations from others
A thorough literature search through Cochrane Database Systematic Reviews, AHRQ, National Guideline Clearing-house, and Medline did not yield any guidelines or consensus statements from other organizations or specialty groups on the evaluation or treatment of night sweats.
1. Mold JW, Mathew MK, Belgore S, Dehaven M. Prevalence of night sweats in primary care patients: an OKPRN and TAFP-Net collaborative study. J Fam Pract 2002;51:452-456.
2. Mold JW, Roberts M, Aboshady HM. Prevalence and predictors of night sweats, day sweats, and hot flashes in older primary care patients: an OKPRN study. Ann Fam Med 2004;2:391-397.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? J Clin Gastroenenterol 1989;11:590-591.
4. Smetana GW. Approach to the patient with night sweats. UpToDate [database online]. Updated October 3, 2006. Available at: www.uptodate.com.
5. Viera AJ, Bond MM, Yates SW. Diagnosing night sweats. Am Fam Physician 2003;67:1019-1024.
6. Chambliss ML. What is the appropriate diagnostic approach for patients who complain of night sweats? Arch Fam Med 1999;8:168-169.
7. MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Datab Syst Rev 2004;CD002978.-
8. Williamson J, White A, Hart A, Ernst E. Randomised controlled trial of reflexology for menopausal symptoms. BJOG 2002;109:1050-1055.
1. Mold JW, Mathew MK, Belgore S, Dehaven M. Prevalence of night sweats in primary care patients: an OKPRN and TAFP-Net collaborative study. J Fam Pract 2002;51:452-456.
2. Mold JW, Roberts M, Aboshady HM. Prevalence and predictors of night sweats, day sweats, and hot flashes in older primary care patients: an OKPRN study. Ann Fam Med 2004;2:391-397.
3. Reynolds WA. Are night sweats a sign of esophageal reflux? J Clin Gastroenenterol 1989;11:590-591.
4. Smetana GW. Approach to the patient with night sweats. UpToDate [database online]. Updated October 3, 2006. Available at: www.uptodate.com.
5. Viera AJ, Bond MM, Yates SW. Diagnosing night sweats. Am Fam Physician 2003;67:1019-1024.
6. Chambliss ML. What is the appropriate diagnostic approach for patients who complain of night sweats? Arch Fam Med 1999;8:168-169.
7. MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Datab Syst Rev 2004;CD002978.-
8. Williamson J, White A, Hart A, Ernst E. Randomised controlled trial of reflexology for menopausal symptoms. BJOG 2002;109:1050-1055.
Evidence-based answers from the Family Physicians Inquiries Network
Are breast self-exams or clinical exams effective for screening breast cancer?
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
Evidence-based answers from the Family Physicians Inquiries Network