User login
Retrospective Review on the Safety and Efficacy of Direct Oral Anticoagulants Compared With Warfarin in Patients With Cirrhosis
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
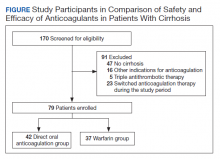
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
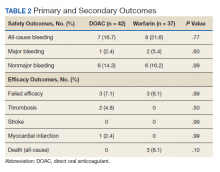
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
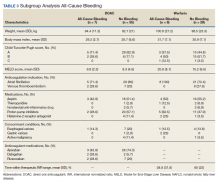
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507