User login
Postpartum thrombosis risk high for 12 weeks
SAN DIEGO – The risk of thrombosis after giving birth remains significantly elevated for 12 weeks after delivery, twice as long as previously thought, an analysis of data on 1.7 million women found.
Dr. Hooman Kamel and his associates analyzed data on 1,687,930 million women admitted to nonfederal acute-care hospitals or emergency departments in California for first-time labor and delivery during 2005-2010, 1,015 of whom had a thrombotic event within 24 weeks after delivery (0.06%). These included strokes (248), MIs (47), and venous thromboemboli (720 cases).
The chance of a clotting event was 11-fold higher than normal in the first 6 weeks after delivery and was doubled when compared with normal in postpartum weeks 7-12, he reported in a press briefing at the International Stroke Conference.
An extra 22 cases/100,000 deliveries occurred in the first 6 weeks postpartum and an extra 3 cases per 100,000 deliveries occurred in weeks 7-12 postpartum.
By 13-18 weeks after delivery, a 40% higher odds of blood clot was not significantly different than with a year later, said Dr. Kamel, a neurologist at Cornell University, N.Y.
The blood clot risk returned to normal levels seen in women who’ve never delivered a baby by 19-24 weeks after delivery.
The New England Journal of Medicine published the findings online (2014 Feb. 13 [doi: 10.1056/NEJMoa1311485]).
The study confirms that thrombosis after delivery is rare and suggests that clinicians may want to take seriously any possible symptoms of stroke beyond the period that’s generally thought of as postpartum: 6 weeks after delivery. Especially in women who have other risk factors for thrombosis, awareness of continued elevated risk in weeks 7-12 could lead to quicker treatment if a blood clot forms, he said in an interview at the meeting, sponsored by the American Heart Association.
Current guidelines call for postpartum blood-thinning therapy in high-risk women who had an elevated stroke risk prior to pregnancy or other risk factors, and it’s unclear whether this should be extended beyond 6 weeks postpartum, Dr. Kamel said. The new findings may prompt research in this direction.
Meanwhile, physicians and women should take seriously any symptoms of possible thrombosis out to 12 weeks after delivery, such as chest pain or pressure, he said.
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Kamel and his colleagues reported having no financial disclosures.
On Twitter @sherryboschert
Correction, 3/4/2014: An earlier version of this article misstated the amount of time passed between delivery and blood clot formation.
SAN DIEGO – The risk of thrombosis after giving birth remains significantly elevated for 12 weeks after delivery, twice as long as previously thought, an analysis of data on 1.7 million women found.
Dr. Hooman Kamel and his associates analyzed data on 1,687,930 million women admitted to nonfederal acute-care hospitals or emergency departments in California for first-time labor and delivery during 2005-2010, 1,015 of whom had a thrombotic event within 24 weeks after delivery (0.06%). These included strokes (248), MIs (47), and venous thromboemboli (720 cases).
The chance of a clotting event was 11-fold higher than normal in the first 6 weeks after delivery and was doubled when compared with normal in postpartum weeks 7-12, he reported in a press briefing at the International Stroke Conference.
An extra 22 cases/100,000 deliveries occurred in the first 6 weeks postpartum and an extra 3 cases per 100,000 deliveries occurred in weeks 7-12 postpartum.
By 13-18 weeks after delivery, a 40% higher odds of blood clot was not significantly different than with a year later, said Dr. Kamel, a neurologist at Cornell University, N.Y.
The blood clot risk returned to normal levels seen in women who’ve never delivered a baby by 19-24 weeks after delivery.
The New England Journal of Medicine published the findings online (2014 Feb. 13 [doi: 10.1056/NEJMoa1311485]).
The study confirms that thrombosis after delivery is rare and suggests that clinicians may want to take seriously any possible symptoms of stroke beyond the period that’s generally thought of as postpartum: 6 weeks after delivery. Especially in women who have other risk factors for thrombosis, awareness of continued elevated risk in weeks 7-12 could lead to quicker treatment if a blood clot forms, he said in an interview at the meeting, sponsored by the American Heart Association.
Current guidelines call for postpartum blood-thinning therapy in high-risk women who had an elevated stroke risk prior to pregnancy or other risk factors, and it’s unclear whether this should be extended beyond 6 weeks postpartum, Dr. Kamel said. The new findings may prompt research in this direction.
Meanwhile, physicians and women should take seriously any symptoms of possible thrombosis out to 12 weeks after delivery, such as chest pain or pressure, he said.
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Kamel and his colleagues reported having no financial disclosures.
On Twitter @sherryboschert
Correction, 3/4/2014: An earlier version of this article misstated the amount of time passed between delivery and blood clot formation.
SAN DIEGO – The risk of thrombosis after giving birth remains significantly elevated for 12 weeks after delivery, twice as long as previously thought, an analysis of data on 1.7 million women found.
Dr. Hooman Kamel and his associates analyzed data on 1,687,930 million women admitted to nonfederal acute-care hospitals or emergency departments in California for first-time labor and delivery during 2005-2010, 1,015 of whom had a thrombotic event within 24 weeks after delivery (0.06%). These included strokes (248), MIs (47), and venous thromboemboli (720 cases).
The chance of a clotting event was 11-fold higher than normal in the first 6 weeks after delivery and was doubled when compared with normal in postpartum weeks 7-12, he reported in a press briefing at the International Stroke Conference.
An extra 22 cases/100,000 deliveries occurred in the first 6 weeks postpartum and an extra 3 cases per 100,000 deliveries occurred in weeks 7-12 postpartum.
By 13-18 weeks after delivery, a 40% higher odds of blood clot was not significantly different than with a year later, said Dr. Kamel, a neurologist at Cornell University, N.Y.
The blood clot risk returned to normal levels seen in women who’ve never delivered a baby by 19-24 weeks after delivery.
The New England Journal of Medicine published the findings online (2014 Feb. 13 [doi: 10.1056/NEJMoa1311485]).
The study confirms that thrombosis after delivery is rare and suggests that clinicians may want to take seriously any possible symptoms of stroke beyond the period that’s generally thought of as postpartum: 6 weeks after delivery. Especially in women who have other risk factors for thrombosis, awareness of continued elevated risk in weeks 7-12 could lead to quicker treatment if a blood clot forms, he said in an interview at the meeting, sponsored by the American Heart Association.
Current guidelines call for postpartum blood-thinning therapy in high-risk women who had an elevated stroke risk prior to pregnancy or other risk factors, and it’s unclear whether this should be extended beyond 6 weeks postpartum, Dr. Kamel said. The new findings may prompt research in this direction.
Meanwhile, physicians and women should take seriously any symptoms of possible thrombosis out to 12 weeks after delivery, such as chest pain or pressure, he said.
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Kamel and his colleagues reported having no financial disclosures.
On Twitter @sherryboschert
Correction, 3/4/2014: An earlier version of this article misstated the amount of time passed between delivery and blood clot formation.
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: The odds of developing a thrombosis remained significantly elevated for 12 weeks postpartum: 11-fold higher during weeks 0-6 and two times higher in weeks 7-12, compared with 1 year after pregnancy.
Data source: A retrospective analysis of data on 1.7 million pregnant women at California hospitals, 1,015 of whom developed blood clots within 24 weeks* of delivery.
Disclosures: The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Kamel and his colleagues reported having no financial disclosures.
Treat subclinical hypothyroidism in pregnancy, expert says
SAN FRANCISCO – Pregnancy is one of the few relatively clear reasons to screen and treat for subclinical hypothyroidism, according to Dr. Elizabeth J. Murphy.
There are reasons to consider treating subclinical hypothyroidism in women other than pregnancy, but there’s no clear right or wrong answer in most cases, she said at a conference on women’s health sponsored by the University of California, San Francisco.
The Endocrine Society in 2012 recommended against universal thyroid screening of healthy women before pregnancy and said to screen those who are at high risk of thyroid disease, but they rated the evidence for those recommendations as poor (J. Clin. Endocrinol. Metab. 2012;97:2543-65).
For newly pregnant women, the guidelines offered a choice of two options, Dr. Murphy said: Screen all pregnant women by week 9 or at the first prenatal visit (with fair evidence to support this strategy), or screen only high-risk women unless that’s too burdensome, in which case, screen all pregnant women (with poor evidence behind this).
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice issued Opinion No. 381 in 2007 (and reaffirmed it in 2012) saying that routine screening for subclinical hypothyroidism is not currently recommended because there’s no evidence that routinely identifying and treating pregnant women with subclinical hypothyroidism improves outcomes (Obstet. Gynecol. 2007;110:959-60).
"I don’t agree with any of those, and I’m not the only one who doesn’t agree," said Dr. Murphy, chief of the division of endocrinology at San Francisco General Hospital.
A study of serum samples from 25,216 pregnant women and follow-up on their children at ages 7-9 years found lower IQ scores in the offspring of women with undiagnosed hypothyroidism during pregnancy, compared with women whose hypothyroidism was treated prior to pregnancy or euthyroid women in a control group (N. Engl. J. Med. 1999;341:549-55).
A more recent multinational, randomized trial of 21,846 women who were screened at a median of 12 weeks and 3 days of gestation reported that identifying and treating hypothyroidism did not improve the cognitive function of offspring at age 3, compared with a control group whose serum was analyzed after delivery (N. Engl. J. Med. 2012;366:493-501). That study was flawed, however, and "doesn’t provide useful data for prepregnancy screening. It only provides data for screening at 12 weeks," Dr. Murphy said.
The fetal thyroid develops at week 12, and the mother’s thyroid function needs to be in good shape before then, she explained. In addition, the median thyroid-stimulating hormone (TSH) level in the study was low, and half of the women in the study were enrolled not because they had a high TSH level but because they had a low level of free T4, an assay that is "notoriously unreliable in pregnancy," she said. "Many people recommend getting a total T4." It’s unclear whether many of the women who were considered to be hypothyroid truly were. Lastly, 3 years of age might be too young to assess cognitive function in the offspring, she said.
A huge study on the same subject is underway in China with plans to screen 21,500 pregnant women and treat 4,800 for hypothyroidism before pregnancy, she said.
A separate study suggested that both universal thyroid screening in pregnancy and risk-based screening are cost effective, compared with no screening (J. Clin. Endocrinol. Metabol. 2012;97:1536-46).
Guidelines from the American Thyroid Association in 2011 recommended treating pregnant women if the TSH level is greater than 10 mIU/L or if the patient is positive for thyroid peroxidase antibody (Thyroid 2011;21:1081-125). Most clinicians, however, probably would want to treat a woman in early pregnancy whose TSH level is 9 mIU/L, "especially with the IQ data that we have out there," Dr. Murphy said. "Congenital hypothyroidism, remember, is cretinism. It’s really good to have thyroid when your brain’s developing. I would definitely treat a woman with subclinical hypothyroidism who is pregnant."
Dr. Murphy reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Pregnancy is one of the few relatively clear reasons to screen and treat for subclinical hypothyroidism, according to Dr. Elizabeth J. Murphy.
There are reasons to consider treating subclinical hypothyroidism in women other than pregnancy, but there’s no clear right or wrong answer in most cases, she said at a conference on women’s health sponsored by the University of California, San Francisco.
The Endocrine Society in 2012 recommended against universal thyroid screening of healthy women before pregnancy and said to screen those who are at high risk of thyroid disease, but they rated the evidence for those recommendations as poor (J. Clin. Endocrinol. Metab. 2012;97:2543-65).
For newly pregnant women, the guidelines offered a choice of two options, Dr. Murphy said: Screen all pregnant women by week 9 or at the first prenatal visit (with fair evidence to support this strategy), or screen only high-risk women unless that’s too burdensome, in which case, screen all pregnant women (with poor evidence behind this).
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice issued Opinion No. 381 in 2007 (and reaffirmed it in 2012) saying that routine screening for subclinical hypothyroidism is not currently recommended because there’s no evidence that routinely identifying and treating pregnant women with subclinical hypothyroidism improves outcomes (Obstet. Gynecol. 2007;110:959-60).
"I don’t agree with any of those, and I’m not the only one who doesn’t agree," said Dr. Murphy, chief of the division of endocrinology at San Francisco General Hospital.
A study of serum samples from 25,216 pregnant women and follow-up on their children at ages 7-9 years found lower IQ scores in the offspring of women with undiagnosed hypothyroidism during pregnancy, compared with women whose hypothyroidism was treated prior to pregnancy or euthyroid women in a control group (N. Engl. J. Med. 1999;341:549-55).
A more recent multinational, randomized trial of 21,846 women who were screened at a median of 12 weeks and 3 days of gestation reported that identifying and treating hypothyroidism did not improve the cognitive function of offspring at age 3, compared with a control group whose serum was analyzed after delivery (N. Engl. J. Med. 2012;366:493-501). That study was flawed, however, and "doesn’t provide useful data for prepregnancy screening. It only provides data for screening at 12 weeks," Dr. Murphy said.
The fetal thyroid develops at week 12, and the mother’s thyroid function needs to be in good shape before then, she explained. In addition, the median thyroid-stimulating hormone (TSH) level in the study was low, and half of the women in the study were enrolled not because they had a high TSH level but because they had a low level of free T4, an assay that is "notoriously unreliable in pregnancy," she said. "Many people recommend getting a total T4." It’s unclear whether many of the women who were considered to be hypothyroid truly were. Lastly, 3 years of age might be too young to assess cognitive function in the offspring, she said.
A huge study on the same subject is underway in China with plans to screen 21,500 pregnant women and treat 4,800 for hypothyroidism before pregnancy, she said.
A separate study suggested that both universal thyroid screening in pregnancy and risk-based screening are cost effective, compared with no screening (J. Clin. Endocrinol. Metabol. 2012;97:1536-46).
Guidelines from the American Thyroid Association in 2011 recommended treating pregnant women if the TSH level is greater than 10 mIU/L or if the patient is positive for thyroid peroxidase antibody (Thyroid 2011;21:1081-125). Most clinicians, however, probably would want to treat a woman in early pregnancy whose TSH level is 9 mIU/L, "especially with the IQ data that we have out there," Dr. Murphy said. "Congenital hypothyroidism, remember, is cretinism. It’s really good to have thyroid when your brain’s developing. I would definitely treat a woman with subclinical hypothyroidism who is pregnant."
Dr. Murphy reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Pregnancy is one of the few relatively clear reasons to screen and treat for subclinical hypothyroidism, according to Dr. Elizabeth J. Murphy.
There are reasons to consider treating subclinical hypothyroidism in women other than pregnancy, but there’s no clear right or wrong answer in most cases, she said at a conference on women’s health sponsored by the University of California, San Francisco.
The Endocrine Society in 2012 recommended against universal thyroid screening of healthy women before pregnancy and said to screen those who are at high risk of thyroid disease, but they rated the evidence for those recommendations as poor (J. Clin. Endocrinol. Metab. 2012;97:2543-65).
For newly pregnant women, the guidelines offered a choice of two options, Dr. Murphy said: Screen all pregnant women by week 9 or at the first prenatal visit (with fair evidence to support this strategy), or screen only high-risk women unless that’s too burdensome, in which case, screen all pregnant women (with poor evidence behind this).
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice issued Opinion No. 381 in 2007 (and reaffirmed it in 2012) saying that routine screening for subclinical hypothyroidism is not currently recommended because there’s no evidence that routinely identifying and treating pregnant women with subclinical hypothyroidism improves outcomes (Obstet. Gynecol. 2007;110:959-60).
"I don’t agree with any of those, and I’m not the only one who doesn’t agree," said Dr. Murphy, chief of the division of endocrinology at San Francisco General Hospital.
A study of serum samples from 25,216 pregnant women and follow-up on their children at ages 7-9 years found lower IQ scores in the offspring of women with undiagnosed hypothyroidism during pregnancy, compared with women whose hypothyroidism was treated prior to pregnancy or euthyroid women in a control group (N. Engl. J. Med. 1999;341:549-55).
A more recent multinational, randomized trial of 21,846 women who were screened at a median of 12 weeks and 3 days of gestation reported that identifying and treating hypothyroidism did not improve the cognitive function of offspring at age 3, compared with a control group whose serum was analyzed after delivery (N. Engl. J. Med. 2012;366:493-501). That study was flawed, however, and "doesn’t provide useful data for prepregnancy screening. It only provides data for screening at 12 weeks," Dr. Murphy said.
The fetal thyroid develops at week 12, and the mother’s thyroid function needs to be in good shape before then, she explained. In addition, the median thyroid-stimulating hormone (TSH) level in the study was low, and half of the women in the study were enrolled not because they had a high TSH level but because they had a low level of free T4, an assay that is "notoriously unreliable in pregnancy," she said. "Many people recommend getting a total T4." It’s unclear whether many of the women who were considered to be hypothyroid truly were. Lastly, 3 years of age might be too young to assess cognitive function in the offspring, she said.
A huge study on the same subject is underway in China with plans to screen 21,500 pregnant women and treat 4,800 for hypothyroidism before pregnancy, she said.
A separate study suggested that both universal thyroid screening in pregnancy and risk-based screening are cost effective, compared with no screening (J. Clin. Endocrinol. Metabol. 2012;97:1536-46).
Guidelines from the American Thyroid Association in 2011 recommended treating pregnant women if the TSH level is greater than 10 mIU/L or if the patient is positive for thyroid peroxidase antibody (Thyroid 2011;21:1081-125). Most clinicians, however, probably would want to treat a woman in early pregnancy whose TSH level is 9 mIU/L, "especially with the IQ data that we have out there," Dr. Murphy said. "Congenital hypothyroidism, remember, is cretinism. It’s really good to have thyroid when your brain’s developing. I would definitely treat a woman with subclinical hypothyroidism who is pregnant."
Dr. Murphy reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM A CONFERENCE ON WOMEN’S HEALTH
Sepsis less common, less deadly in pregnancy
SAN FRANCISCO – The incidence of severe sepsis was nearly five times lower and the risk of death from severe sepsis was 43% lower during pregnancy, compared with nonpregnant women, in a large retrospective study of data on more than 47 million pregnancy-related discharges.
The decreased mortality rate in pregnancy remained after investigators controlled for the effects of age, comorbidities, and severity of illness, Dr. Gagan Kumar reported.
The incidence of sepsis in pregnancy increased fourfold from 2000 to 2009 – from 0.01% to 0.04% – and tripled in nonpregnant women – from 0.06% to 0.18% – while the U.S. pregnancy rate and the rate of hospitalizations during pregnancy remained relatively stable during that period, he said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In-hospital mortality from severe sepsis decreased gradually for nonpregnant patients from about 30% in 2000 to approximately 18% in 2009, but remained relatively stable in pregnant patients, increasing from approximately 3% in 2000 to 10% in 2009, he said.
The investigators studied claims data from 2000 to 2009 from the Nationwide Inpatient Sample for 47,027,806 pregnancy-related discharges of women aged 15-44 years. Of these, 0.03% had a diagnosis of severe sepsis. Among all cases of severe sepsis in the cohort, 2.4% were during pregnancy (643,417 cases).
Eight percent of pregnant women with severe sepsis died, compared with 22% of nonpregnant women with severe sepsis, a significant difference. The median time to death was significantly longer in pregnancy (10 days) than without pregnancy (8.5 days). The median length of stay was significantly shorter in pregnant women who survived severe sepsis (8 days), compared with nonpregnant survivors (11 days). Pregnant survivors were more likely to be discharged home and less likely to go to a skilled nursing facility or have home care compared with nonpregnant survivors, reported Dr. Kumar of the Medical College of Wisconsin, Milwaukee.
Compared with nonpregnant women, pregnant women were significantly less likely to have three or more organs fail (16% vs. 22%) or to have cardiac, renal, hepatic, hematologic, metabolic, or neurologic failure. Pregnant women were significantly more likely to have respiratory failure than were nonpregnant women.
The likelihood of dying of severe sepsis was 62% lower in pregnant women, compared with nonpregnant women, in an unadjusted analysis and 41%-43% lower than in nonpregnant women under three separate analyses that adjusted for various risk factors or incorporated matched data, Dr. Kumar said.
Although the rate of in-hospital mortality decreased in nonpregnant women from approximately 30% in 2000 to approximately 18% in 2009, the rate increased in pregnant women from approximately 4% in 2000 to 10% in 2009.
Published data are limited and suggested that sepsis in pregnancy is rare, affecting approximately 0.1% of pregnancies, with septic shock in approximately 0.01%-0.001%. These studies relied predominantly on single centers, and used varying definitions of severe sepsis; most were conducted before the year 2000. Since then, the average age of mothers in pregnancy has risen, invasive tests are more common, the rate of cesarean deliveries increased by 7% per year between 1996 and 2011, and the rates of comorbidities such as obesity and diabetes during pregnancy have increased, he said.
Pregnant patients with severe sepsis were 7 years younger on average (27 years of age), compared with nonpregnant women with severe sepsis (age 34 years). Severe sepsis in pregnancy was significantly more common in Hispanics (17%) and Asians (4%), compared with nonpregnant patients (9% and 2%, respectively). Pregnant patients with severe sepsis also had less comorbidity, obesity, and atrial fibrillation, compared with nonpregnant patients with severe sepsis.
Previously Dr. Kumar and his associates reported that the rate of hospitalizations for severe sepsis increased from 143/100,000 persons in 2000 to 343/100,000 in 2007, while mortality rates from severe sepsis decreased from 39% to 27% (Chest 2011;40:1223-31).
The findings of the current study raise questions worth pursuing in future studies, he said, such as the reasons for rising rates of severe sepsis in pregnancy despite no increase in pregnancies or hospitalizations during pregnancy. Pregnancy is considered an immunocompromised state, so why are incidence and mortality rates for severe sepsis lower in pregnancy? he asked. And why has mortality from severe sepsis in pregnancy not improved over the past 10 years?
Dr. Kumar reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The incidence of severe sepsis was nearly five times lower and the risk of death from severe sepsis was 43% lower during pregnancy, compared with nonpregnant women, in a large retrospective study of data on more than 47 million pregnancy-related discharges.
The decreased mortality rate in pregnancy remained after investigators controlled for the effects of age, comorbidities, and severity of illness, Dr. Gagan Kumar reported.
The incidence of sepsis in pregnancy increased fourfold from 2000 to 2009 – from 0.01% to 0.04% – and tripled in nonpregnant women – from 0.06% to 0.18% – while the U.S. pregnancy rate and the rate of hospitalizations during pregnancy remained relatively stable during that period, he said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In-hospital mortality from severe sepsis decreased gradually for nonpregnant patients from about 30% in 2000 to approximately 18% in 2009, but remained relatively stable in pregnant patients, increasing from approximately 3% in 2000 to 10% in 2009, he said.
The investigators studied claims data from 2000 to 2009 from the Nationwide Inpatient Sample for 47,027,806 pregnancy-related discharges of women aged 15-44 years. Of these, 0.03% had a diagnosis of severe sepsis. Among all cases of severe sepsis in the cohort, 2.4% were during pregnancy (643,417 cases).
Eight percent of pregnant women with severe sepsis died, compared with 22% of nonpregnant women with severe sepsis, a significant difference. The median time to death was significantly longer in pregnancy (10 days) than without pregnancy (8.5 days). The median length of stay was significantly shorter in pregnant women who survived severe sepsis (8 days), compared with nonpregnant survivors (11 days). Pregnant survivors were more likely to be discharged home and less likely to go to a skilled nursing facility or have home care compared with nonpregnant survivors, reported Dr. Kumar of the Medical College of Wisconsin, Milwaukee.
Compared with nonpregnant women, pregnant women were significantly less likely to have three or more organs fail (16% vs. 22%) or to have cardiac, renal, hepatic, hematologic, metabolic, or neurologic failure. Pregnant women were significantly more likely to have respiratory failure than were nonpregnant women.
The likelihood of dying of severe sepsis was 62% lower in pregnant women, compared with nonpregnant women, in an unadjusted analysis and 41%-43% lower than in nonpregnant women under three separate analyses that adjusted for various risk factors or incorporated matched data, Dr. Kumar said.
Although the rate of in-hospital mortality decreased in nonpregnant women from approximately 30% in 2000 to approximately 18% in 2009, the rate increased in pregnant women from approximately 4% in 2000 to 10% in 2009.
Published data are limited and suggested that sepsis in pregnancy is rare, affecting approximately 0.1% of pregnancies, with septic shock in approximately 0.01%-0.001%. These studies relied predominantly on single centers, and used varying definitions of severe sepsis; most were conducted before the year 2000. Since then, the average age of mothers in pregnancy has risen, invasive tests are more common, the rate of cesarean deliveries increased by 7% per year between 1996 and 2011, and the rates of comorbidities such as obesity and diabetes during pregnancy have increased, he said.
Pregnant patients with severe sepsis were 7 years younger on average (27 years of age), compared with nonpregnant women with severe sepsis (age 34 years). Severe sepsis in pregnancy was significantly more common in Hispanics (17%) and Asians (4%), compared with nonpregnant patients (9% and 2%, respectively). Pregnant patients with severe sepsis also had less comorbidity, obesity, and atrial fibrillation, compared with nonpregnant patients with severe sepsis.
Previously Dr. Kumar and his associates reported that the rate of hospitalizations for severe sepsis increased from 143/100,000 persons in 2000 to 343/100,000 in 2007, while mortality rates from severe sepsis decreased from 39% to 27% (Chest 2011;40:1223-31).
The findings of the current study raise questions worth pursuing in future studies, he said, such as the reasons for rising rates of severe sepsis in pregnancy despite no increase in pregnancies or hospitalizations during pregnancy. Pregnancy is considered an immunocompromised state, so why are incidence and mortality rates for severe sepsis lower in pregnancy? he asked. And why has mortality from severe sepsis in pregnancy not improved over the past 10 years?
Dr. Kumar reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The incidence of severe sepsis was nearly five times lower and the risk of death from severe sepsis was 43% lower during pregnancy, compared with nonpregnant women, in a large retrospective study of data on more than 47 million pregnancy-related discharges.
The decreased mortality rate in pregnancy remained after investigators controlled for the effects of age, comorbidities, and severity of illness, Dr. Gagan Kumar reported.
The incidence of sepsis in pregnancy increased fourfold from 2000 to 2009 – from 0.01% to 0.04% – and tripled in nonpregnant women – from 0.06% to 0.18% – while the U.S. pregnancy rate and the rate of hospitalizations during pregnancy remained relatively stable during that period, he said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In-hospital mortality from severe sepsis decreased gradually for nonpregnant patients from about 30% in 2000 to approximately 18% in 2009, but remained relatively stable in pregnant patients, increasing from approximately 3% in 2000 to 10% in 2009, he said.
The investigators studied claims data from 2000 to 2009 from the Nationwide Inpatient Sample for 47,027,806 pregnancy-related discharges of women aged 15-44 years. Of these, 0.03% had a diagnosis of severe sepsis. Among all cases of severe sepsis in the cohort, 2.4% were during pregnancy (643,417 cases).
Eight percent of pregnant women with severe sepsis died, compared with 22% of nonpregnant women with severe sepsis, a significant difference. The median time to death was significantly longer in pregnancy (10 days) than without pregnancy (8.5 days). The median length of stay was significantly shorter in pregnant women who survived severe sepsis (8 days), compared with nonpregnant survivors (11 days). Pregnant survivors were more likely to be discharged home and less likely to go to a skilled nursing facility or have home care compared with nonpregnant survivors, reported Dr. Kumar of the Medical College of Wisconsin, Milwaukee.
Compared with nonpregnant women, pregnant women were significantly less likely to have three or more organs fail (16% vs. 22%) or to have cardiac, renal, hepatic, hematologic, metabolic, or neurologic failure. Pregnant women were significantly more likely to have respiratory failure than were nonpregnant women.
The likelihood of dying of severe sepsis was 62% lower in pregnant women, compared with nonpregnant women, in an unadjusted analysis and 41%-43% lower than in nonpregnant women under three separate analyses that adjusted for various risk factors or incorporated matched data, Dr. Kumar said.
Although the rate of in-hospital mortality decreased in nonpregnant women from approximately 30% in 2000 to approximately 18% in 2009, the rate increased in pregnant women from approximately 4% in 2000 to 10% in 2009.
Published data are limited and suggested that sepsis in pregnancy is rare, affecting approximately 0.1% of pregnancies, with septic shock in approximately 0.01%-0.001%. These studies relied predominantly on single centers, and used varying definitions of severe sepsis; most were conducted before the year 2000. Since then, the average age of mothers in pregnancy has risen, invasive tests are more common, the rate of cesarean deliveries increased by 7% per year between 1996 and 2011, and the rates of comorbidities such as obesity and diabetes during pregnancy have increased, he said.
Pregnant patients with severe sepsis were 7 years younger on average (27 years of age), compared with nonpregnant women with severe sepsis (age 34 years). Severe sepsis in pregnancy was significantly more common in Hispanics (17%) and Asians (4%), compared with nonpregnant patients (9% and 2%, respectively). Pregnant patients with severe sepsis also had less comorbidity, obesity, and atrial fibrillation, compared with nonpregnant patients with severe sepsis.
Previously Dr. Kumar and his associates reported that the rate of hospitalizations for severe sepsis increased from 143/100,000 persons in 2000 to 343/100,000 in 2007, while mortality rates from severe sepsis decreased from 39% to 27% (Chest 2011;40:1223-31).
The findings of the current study raise questions worth pursuing in future studies, he said, such as the reasons for rising rates of severe sepsis in pregnancy despite no increase in pregnancies or hospitalizations during pregnancy. Pregnancy is considered an immunocompromised state, so why are incidence and mortality rates for severe sepsis lower in pregnancy? he asked. And why has mortality from severe sepsis in pregnancy not improved over the past 10 years?
Dr. Kumar reported having no financial disclosures.
On Twitter @sherryboschert
AT THE CRITICAL CARE CONGRESS
Major finding: The odds of death in women with severe sepsis were 43% lower during pregnancy, compared with nonpregnant women.
Data source: A retrospective analysis of nationwide data on more than 47 million pregnancy-related discharges.
Disclosures: Dr. Kumar reported having no financial disclosures.
Video: Positive trends for outcomes in uric acid stroke trial gives optimism
The outcomes in a double-blind, randomized, placebo-controlled trial in which uric acid was added to thrombolytic therapy for acute ischemic stroke were not statistically significant, but the positive trend seen in the outcomes indicates that a larger trial may show significant benefits, Dr. Angel Chamorro of the Hospital Clinic of the University of Barcelona (Spain) said at the International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The outcomes in a double-blind, randomized, placebo-controlled trial in which uric acid was added to thrombolytic therapy for acute ischemic stroke were not statistically significant, but the positive trend seen in the outcomes indicates that a larger trial may show significant benefits, Dr. Angel Chamorro of the Hospital Clinic of the University of Barcelona (Spain) said at the International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The outcomes in a double-blind, randomized, placebo-controlled trial in which uric acid was added to thrombolytic therapy for acute ischemic stroke were not statistically significant, but the positive trend seen in the outcomes indicates that a larger trial may show significant benefits, Dr. Angel Chamorro of the Hospital Clinic of the University of Barcelona (Spain) said at the International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video: Study hints uric acid reduces acute stroke symptoms
Although the results of a double-blind, randomized, placebo-controlled clinical trial of adding uric acid to thrombolytic therapy hinted at potentially beneficial effects on acute ischemic stroke outcomes, Dr. Steven M. Greenberg of Harvard Medical School, Boston, advised waiting for the results of larger trials that confirm the potential benefits given the poor history behind seemingly promising preclinical therapies for acute stroke.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Although the results of a double-blind, randomized, placebo-controlled clinical trial of adding uric acid to thrombolytic therapy hinted at potentially beneficial effects on acute ischemic stroke outcomes, Dr. Steven M. Greenberg of Harvard Medical School, Boston, advised waiting for the results of larger trials that confirm the potential benefits given the poor history behind seemingly promising preclinical therapies for acute stroke.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Although the results of a double-blind, randomized, placebo-controlled clinical trial of adding uric acid to thrombolytic therapy hinted at potentially beneficial effects on acute ischemic stroke outcomes, Dr. Steven M. Greenberg of Harvard Medical School, Boston, advised waiting for the results of larger trials that confirm the potential benefits given the poor history behind seemingly promising preclinical therapies for acute stroke.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Uric acid for stroke trials to continue
SAN DIEGO – Adding uric acid to thrombolytic therapy for acute stroke looked promising enough in a double-blind placebo-controlled trial of 420 adult patients that investigators will seek funding for a larger trial.
Thirty-nine percent of 211 patients randomized to the uric acid group achieved an "excellent" outcome on measures of symptoms and function 90 days later, but the 200 patients in the placebo group also had a robust response with 33% achieving excellent outcomes, Dr. Angel Chamorro reported at the International Stroke Conference. Nine patients were excluded from the analysis because they didn’t take the medication or were determined not to have a stroke.
The difference between groups did not reach statistical significance, and the study was not powered to assess less than a 14% difference between groups. Safety outcomes were similar between groups, however, and statistical trends toward better outcomes with uric acid in this proof-of-concept trial are enough to justify a study of 1,500 patients to determine whether this is a useful treatment, said Dr. Chamorro, director of the comprehensive stroke center at Hospital Clinic of the University of Barcelona (Spain).
If the results are validated in subsequent trials, it could mean that for every 14 patients given uric acid for acute stroke, 1 patient would become completely free of stroke symptoms, he said in an interview. Uric acid, a potent antioxidant, is thought to help in stroke by inhibiting free radicals that are created during loss of blood flow and that damage brain cells. Uric acid is being explored by other investigators as a treatment for Parkinson’s disease, he added.
All patients in the Urico-ictus Study were treated with recombinant tissue plasminogen activator (rtPA) within 4.5 hours of acute ischemic stroke symptom onset and randomized to receive a single IV infusion of 1 g uric acid dissolved in vehicle or to a control group whose IV infusion contained the vehicle alone (500 mL containing 0.1% lithium carbonate and 5% mannitol). All patients had a baseline National Institutes of Health Stroke Scale (NIHSS) score greater than 6 but less than 25, a modified Rankin Scale (mRS) score no greater than 2 prior to the stroke, and a cranial CT showing the absence of blood in the CNS.
The primary results showed modified Rankin Scale scores of 0-1 (or 2 if premorbid was 2) at 90 days in 39% on uric acid and 33% on placebo (P = .099).
Among secondary outcomes, the median mRS was 2 in the uric acid group and 3 on placebo (P = .045), suggesting a trend toward greater effectiveness with uric acid. A 1-point absolute reduction in median mRS is similar to the effects seen in the first trials of thrombolytic therapy, Dr. Chamorro pointed out.
Patients on uric acid were significantly less likely to show worsening at day 3 compared with the placebo group (3% vs. 9%). The uric acid group showed a "high statistical trend" toward having fully independent functioning at 90 days as assessed by a score greater than 94 on the Barthel Index of Activities of Daily Living, with 48% on uric acid and 41% on placebo reaching this mark, Dr. Chamorro reported at the meeting, sponsored by the American Heart Association.
Thirteen percent on uric acid and 16% on placebo died within 90 days. Gout occurred within 90 days in 0.5% on uric acid and 2% on placebo. The proportions of patients with an intracranial hemorrhage whose NIHSS score worsened by 4 points within the first 36 hours of treatment were 4% in the uric acid group and 3% in the placebo groups. These safety outcomes did not differ significantly between groups.
Preplanned subgroup analyses showed trends toward greater effectiveness of uric acid in women, patients with high blood glucose levels, or those with moderate strokes, compared with placebo.
Oxidative stress in patients with ischemic stroke contributes to brain damage. The antioxidant properties of uric acid proved to be neuroprotective in animal studies. Dr. Chamorro and his associates reported in a previous prognostic study of 881 patients with acute ischemic stroke that each milligram per deciliter increase in serum uric acid was associated with a 12% increase in the odds of a good clinical outcome (Stroke 2002;33:1048-52). Previous animal studies suggest uric acid may help reduce stroke sequelae.
Dr. Chamorro and his coauthors reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Adding uric acid to thrombolytic therapy for acute stroke looked promising enough in a double-blind placebo-controlled trial of 420 adult patients that investigators will seek funding for a larger trial.
Thirty-nine percent of 211 patients randomized to the uric acid group achieved an "excellent" outcome on measures of symptoms and function 90 days later, but the 200 patients in the placebo group also had a robust response with 33% achieving excellent outcomes, Dr. Angel Chamorro reported at the International Stroke Conference. Nine patients were excluded from the analysis because they didn’t take the medication or were determined not to have a stroke.
The difference between groups did not reach statistical significance, and the study was not powered to assess less than a 14% difference between groups. Safety outcomes were similar between groups, however, and statistical trends toward better outcomes with uric acid in this proof-of-concept trial are enough to justify a study of 1,500 patients to determine whether this is a useful treatment, said Dr. Chamorro, director of the comprehensive stroke center at Hospital Clinic of the University of Barcelona (Spain).
If the results are validated in subsequent trials, it could mean that for every 14 patients given uric acid for acute stroke, 1 patient would become completely free of stroke symptoms, he said in an interview. Uric acid, a potent antioxidant, is thought to help in stroke by inhibiting free radicals that are created during loss of blood flow and that damage brain cells. Uric acid is being explored by other investigators as a treatment for Parkinson’s disease, he added.
All patients in the Urico-ictus Study were treated with recombinant tissue plasminogen activator (rtPA) within 4.5 hours of acute ischemic stroke symptom onset and randomized to receive a single IV infusion of 1 g uric acid dissolved in vehicle or to a control group whose IV infusion contained the vehicle alone (500 mL containing 0.1% lithium carbonate and 5% mannitol). All patients had a baseline National Institutes of Health Stroke Scale (NIHSS) score greater than 6 but less than 25, a modified Rankin Scale (mRS) score no greater than 2 prior to the stroke, and a cranial CT showing the absence of blood in the CNS.
The primary results showed modified Rankin Scale scores of 0-1 (or 2 if premorbid was 2) at 90 days in 39% on uric acid and 33% on placebo (P = .099).
Among secondary outcomes, the median mRS was 2 in the uric acid group and 3 on placebo (P = .045), suggesting a trend toward greater effectiveness with uric acid. A 1-point absolute reduction in median mRS is similar to the effects seen in the first trials of thrombolytic therapy, Dr. Chamorro pointed out.
Patients on uric acid were significantly less likely to show worsening at day 3 compared with the placebo group (3% vs. 9%). The uric acid group showed a "high statistical trend" toward having fully independent functioning at 90 days as assessed by a score greater than 94 on the Barthel Index of Activities of Daily Living, with 48% on uric acid and 41% on placebo reaching this mark, Dr. Chamorro reported at the meeting, sponsored by the American Heart Association.
Thirteen percent on uric acid and 16% on placebo died within 90 days. Gout occurred within 90 days in 0.5% on uric acid and 2% on placebo. The proportions of patients with an intracranial hemorrhage whose NIHSS score worsened by 4 points within the first 36 hours of treatment were 4% in the uric acid group and 3% in the placebo groups. These safety outcomes did not differ significantly between groups.
Preplanned subgroup analyses showed trends toward greater effectiveness of uric acid in women, patients with high blood glucose levels, or those with moderate strokes, compared with placebo.
Oxidative stress in patients with ischemic stroke contributes to brain damage. The antioxidant properties of uric acid proved to be neuroprotective in animal studies. Dr. Chamorro and his associates reported in a previous prognostic study of 881 patients with acute ischemic stroke that each milligram per deciliter increase in serum uric acid was associated with a 12% increase in the odds of a good clinical outcome (Stroke 2002;33:1048-52). Previous animal studies suggest uric acid may help reduce stroke sequelae.
Dr. Chamorro and his coauthors reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Adding uric acid to thrombolytic therapy for acute stroke looked promising enough in a double-blind placebo-controlled trial of 420 adult patients that investigators will seek funding for a larger trial.
Thirty-nine percent of 211 patients randomized to the uric acid group achieved an "excellent" outcome on measures of symptoms and function 90 days later, but the 200 patients in the placebo group also had a robust response with 33% achieving excellent outcomes, Dr. Angel Chamorro reported at the International Stroke Conference. Nine patients were excluded from the analysis because they didn’t take the medication or were determined not to have a stroke.
The difference between groups did not reach statistical significance, and the study was not powered to assess less than a 14% difference between groups. Safety outcomes were similar between groups, however, and statistical trends toward better outcomes with uric acid in this proof-of-concept trial are enough to justify a study of 1,500 patients to determine whether this is a useful treatment, said Dr. Chamorro, director of the comprehensive stroke center at Hospital Clinic of the University of Barcelona (Spain).
If the results are validated in subsequent trials, it could mean that for every 14 patients given uric acid for acute stroke, 1 patient would become completely free of stroke symptoms, he said in an interview. Uric acid, a potent antioxidant, is thought to help in stroke by inhibiting free radicals that are created during loss of blood flow and that damage brain cells. Uric acid is being explored by other investigators as a treatment for Parkinson’s disease, he added.
All patients in the Urico-ictus Study were treated with recombinant tissue plasminogen activator (rtPA) within 4.5 hours of acute ischemic stroke symptom onset and randomized to receive a single IV infusion of 1 g uric acid dissolved in vehicle or to a control group whose IV infusion contained the vehicle alone (500 mL containing 0.1% lithium carbonate and 5% mannitol). All patients had a baseline National Institutes of Health Stroke Scale (NIHSS) score greater than 6 but less than 25, a modified Rankin Scale (mRS) score no greater than 2 prior to the stroke, and a cranial CT showing the absence of blood in the CNS.
The primary results showed modified Rankin Scale scores of 0-1 (or 2 if premorbid was 2) at 90 days in 39% on uric acid and 33% on placebo (P = .099).
Among secondary outcomes, the median mRS was 2 in the uric acid group and 3 on placebo (P = .045), suggesting a trend toward greater effectiveness with uric acid. A 1-point absolute reduction in median mRS is similar to the effects seen in the first trials of thrombolytic therapy, Dr. Chamorro pointed out.
Patients on uric acid were significantly less likely to show worsening at day 3 compared with the placebo group (3% vs. 9%). The uric acid group showed a "high statistical trend" toward having fully independent functioning at 90 days as assessed by a score greater than 94 on the Barthel Index of Activities of Daily Living, with 48% on uric acid and 41% on placebo reaching this mark, Dr. Chamorro reported at the meeting, sponsored by the American Heart Association.
Thirteen percent on uric acid and 16% on placebo died within 90 days. Gout occurred within 90 days in 0.5% on uric acid and 2% on placebo. The proportions of patients with an intracranial hemorrhage whose NIHSS score worsened by 4 points within the first 36 hours of treatment were 4% in the uric acid group and 3% in the placebo groups. These safety outcomes did not differ significantly between groups.
Preplanned subgroup analyses showed trends toward greater effectiveness of uric acid in women, patients with high blood glucose levels, or those with moderate strokes, compared with placebo.
Oxidative stress in patients with ischemic stroke contributes to brain damage. The antioxidant properties of uric acid proved to be neuroprotective in animal studies. Dr. Chamorro and his associates reported in a previous prognostic study of 881 patients with acute ischemic stroke that each milligram per deciliter increase in serum uric acid was associated with a 12% increase in the odds of a good clinical outcome (Stroke 2002;33:1048-52). Previous animal studies suggest uric acid may help reduce stroke sequelae.
Dr. Chamorro and his coauthors reported having no relevant financial disclosures.
On Twitter @sherryboschert
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Thirty-nine percent given uric acid had a modified Rankin Scale score of 0-1 (or 2 if premorbid score was 2) at 90 days, compared with 33% given placebo (P = .099).
Data source: A prospective, randomized, placebo-controlled proof-of-concept trial in 411 adults with acute stroke.
Disclosures: Dr. Chamorro and his coauthors reported having no relevant financial disclosures.
Relatively few in ICUs get end-of-life communication training
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about end-of-life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs (Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities. The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
On Twitter @sherryboschert
I am not at all surprised, nor am I disappointed by these findings. As a nation we are headed in the right direction with improving communication around end-of-life (EOL) issues.
 |
|
One of the recommendations coming from 2008 guidelines by the American College of Physicians has to do with communicating advance directives and addressing the EOL topic with our patients. I am thrilled that we are beginning to have guidelines and recommendations like these to use as stimulation and leverage, improving the patient experience. If we reflect on some relatively depressing data from the last few years looking at internal medicine physicians at the University of California, San Francisco, admitting acutely ill patients and having advance directive discussions with them (J. Gen. Intern. Med. 2011;26:359-66), then I am encouraged by the findings in this ICU study. While the patient population (medical floor vs. ICU) is somewhat different, both populations benefit from advance care planning.
Barriers to end-of-life communications in ICUs include deficits in communication skills and a lack of time. The average amount of time, conservatively, is 45 minutes for these discussions. Care providers may avoid these discussions because of difficulty with their own emotions, perceiving the family as "difficult," a lack of understanding between the health care provider and the patient or family, and poor compliance. There’s little – if any – reimbursement, space is an issue as there are very few family conference rooms in hospitals, and there is mixed messaging by the numerous teams or specialties involved. The list goes on!
The Gundersen Health System in La Crosse, Wisc., is a good example of how to create a successful system of advance care planning. No one does it better. Also, the "premier programs" listed on the website of the Improving Palliative Care in the ICU (IPAL-ICU) Project are examples of leaders in the field.
Training in advance care planning is part of the education of students and residents at our institution. The University of Texas and Seton Healthcare jointly are creating a medical school in Austin. We plan to have all medical students spend time with our palliative care team and learn these communication skills. We began work on the IPAL-ICU program at two of our large hospitals. We also have put in a proposal to teach these skills to our providers and other providers within our community.
Finally, our program is collaborating with elderly advocacy groups in town to train their nurses and social workers in having upstream discussions with the population they serve so that decisions are addressed before hospitalization.
Dr. Stephen J. Bekanich, codirector of the palliative care program at Seton Healthcare, Austin, Tex., coauthors the Palliatively Speaking blog for Hospitalist News. He reported having no financial disclosures.
I am not at all surprised, nor am I disappointed by these findings. As a nation we are headed in the right direction with improving communication around end-of-life (EOL) issues.
 |
|
One of the recommendations coming from 2008 guidelines by the American College of Physicians has to do with communicating advance directives and addressing the EOL topic with our patients. I am thrilled that we are beginning to have guidelines and recommendations like these to use as stimulation and leverage, improving the patient experience. If we reflect on some relatively depressing data from the last few years looking at internal medicine physicians at the University of California, San Francisco, admitting acutely ill patients and having advance directive discussions with them (J. Gen. Intern. Med. 2011;26:359-66), then I am encouraged by the findings in this ICU study. While the patient population (medical floor vs. ICU) is somewhat different, both populations benefit from advance care planning.
Barriers to end-of-life communications in ICUs include deficits in communication skills and a lack of time. The average amount of time, conservatively, is 45 minutes for these discussions. Care providers may avoid these discussions because of difficulty with their own emotions, perceiving the family as "difficult," a lack of understanding between the health care provider and the patient or family, and poor compliance. There’s little – if any – reimbursement, space is an issue as there are very few family conference rooms in hospitals, and there is mixed messaging by the numerous teams or specialties involved. The list goes on!
The Gundersen Health System in La Crosse, Wisc., is a good example of how to create a successful system of advance care planning. No one does it better. Also, the "premier programs" listed on the website of the Improving Palliative Care in the ICU (IPAL-ICU) Project are examples of leaders in the field.
Training in advance care planning is part of the education of students and residents at our institution. The University of Texas and Seton Healthcare jointly are creating a medical school in Austin. We plan to have all medical students spend time with our palliative care team and learn these communication skills. We began work on the IPAL-ICU program at two of our large hospitals. We also have put in a proposal to teach these skills to our providers and other providers within our community.
Finally, our program is collaborating with elderly advocacy groups in town to train their nurses and social workers in having upstream discussions with the population they serve so that decisions are addressed before hospitalization.
Dr. Stephen J. Bekanich, codirector of the palliative care program at Seton Healthcare, Austin, Tex., coauthors the Palliatively Speaking blog for Hospitalist News. He reported having no financial disclosures.
I am not at all surprised, nor am I disappointed by these findings. As a nation we are headed in the right direction with improving communication around end-of-life (EOL) issues.
 |
|
One of the recommendations coming from 2008 guidelines by the American College of Physicians has to do with communicating advance directives and addressing the EOL topic with our patients. I am thrilled that we are beginning to have guidelines and recommendations like these to use as stimulation and leverage, improving the patient experience. If we reflect on some relatively depressing data from the last few years looking at internal medicine physicians at the University of California, San Francisco, admitting acutely ill patients and having advance directive discussions with them (J. Gen. Intern. Med. 2011;26:359-66), then I am encouraged by the findings in this ICU study. While the patient population (medical floor vs. ICU) is somewhat different, both populations benefit from advance care planning.
Barriers to end-of-life communications in ICUs include deficits in communication skills and a lack of time. The average amount of time, conservatively, is 45 minutes for these discussions. Care providers may avoid these discussions because of difficulty with their own emotions, perceiving the family as "difficult," a lack of understanding between the health care provider and the patient or family, and poor compliance. There’s little – if any – reimbursement, space is an issue as there are very few family conference rooms in hospitals, and there is mixed messaging by the numerous teams or specialties involved. The list goes on!
The Gundersen Health System in La Crosse, Wisc., is a good example of how to create a successful system of advance care planning. No one does it better. Also, the "premier programs" listed on the website of the Improving Palliative Care in the ICU (IPAL-ICU) Project are examples of leaders in the field.
Training in advance care planning is part of the education of students and residents at our institution. The University of Texas and Seton Healthcare jointly are creating a medical school in Austin. We plan to have all medical students spend time with our palliative care team and learn these communication skills. We began work on the IPAL-ICU program at two of our large hospitals. We also have put in a proposal to teach these skills to our providers and other providers within our community.
Finally, our program is collaborating with elderly advocacy groups in town to train their nurses and social workers in having upstream discussions with the population they serve so that decisions are addressed before hospitalization.
Dr. Stephen J. Bekanich, codirector of the palliative care program at Seton Healthcare, Austin, Tex., coauthors the Palliatively Speaking blog for Hospitalist News. He reported having no financial disclosures.
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about end-of-life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs (Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities. The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
On Twitter @sherryboschert
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about end-of-life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs (Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities. The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
On Twitter @sherryboschert
AT THE CRITICAL CARE CONGRESS
Palliative care shortens ICU, hospital stays, review data show
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore.
The findings have been submitted for publication, she said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes. Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
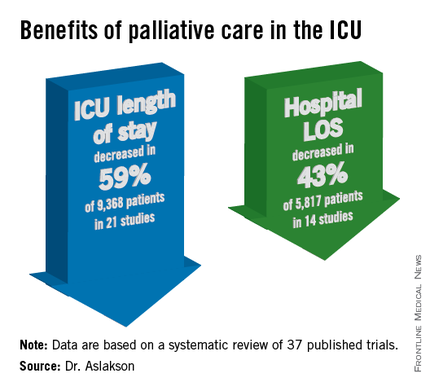
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies. Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson reported.
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said. "No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies. In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of improved communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore.

The findings have been submitted for publication, she said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes. Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies. Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson reported.
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said. "No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies. In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of improved communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore.

The findings have been submitted for publication, she said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes. Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies. Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson reported.
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said. "No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies. In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of improved communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
AT THE CRITICAL CARE CONGRESS
Get same-day ultrasound if pregnant, bleeding
SAN FRANCISCO – Make sure a woman who has uterine bleeding or abdominal pain in the first trimester of pregnancy gets an ultrasound the same day you see her, whether you do the ultrasound yourself, refer her to another physician for a same-day ultrasound, or send her to the emergency department, Dr. Rebecca Jackson advised.
The same-day ultrasound is essential to diagnose ectopic pregnancy early, Dr. Jackson said at a conference on women’s health sponsored by the University of California, San Francisco. The ultrasound is unlikely to show an ectopic pregnancy, but if you see an intrauterine pregnancy, "you’re done," she said.
Ectopic pregnancy occurs in 2% of pregnancies and is the leading cause of U.S. maternal deaths in the first trimester. "The key thing about ectopic pregnancy is that two-thirds of the women dying from an ectopic pregnancy had recently seen a clinician, but had an incorrect or delayed diagnosis," said Dr. Jackson, professor of obstetrics and gynecology at the university and chief of obstetrics and gynecology at San Francisco General Hospital.
If an ultrasound doesn’t show the location of the pregnancy, obtain serial quantitative beta-HCG levels to determine whether the pregnancy is normal or abnormal, she advised. "If it rises appropriately, it’s likely normal, but ectopics can also rise in the same way" as beta-HCG levels with intrauterine pregnancies, she said. "But if it drops or rises very little you know it’s abnormal."
Get a uterine aspiration in women with an abnormal beta-HCG level to look for placental tissue in the uterus, which indicates an intrauterine pregnancy, she said. If there’s no placental tissue in the uterus, treat for ectopic pregnancy.
There’s a shortcut in all of this to keep in mind, she added. If a woman presents with abnormal uterine bleeding or abdominal pain and she tests positive for pregnancy, ask if she desires the pregnancy. If not, you can skip the ultrasound and beta-HCG levels and go straight to the uterine aspiration, Dr. Jackson said.
These steps to diagnosing ectopic pregnancy may sound simple but in reality take ob.gyn. residents years to master. "There are so many flavors of how women present, and you combine that with their desires and their fertility desires, and it’s a really hard thing to manage," she said.
Only approximately 2% of ultrasounds done for possible ectopic pregnancy will show a gestational sac with a yolk sac or fetal pole visible outside the uterus, and thus be diagnostic, Dr. Jackson said. A normal adnexal exam does not exclude an ectopic pregnancy. An empty uterus on ultrasound and a beta-HCG level above the discriminatory zone suggests an ectopic pregnancy, and will be in 86% of cases. An ultrasound showing a complex mass and fluid in a cul-de-sac will be an ectopic pregnancy in 94% of cases. The main role of the ultrasound is to rule in an intrauterine pregnancy.
Diagnosing ectopic pregnancy early decreases the risk of rupture, which has been associated with decreased fertility and increased morbidity and mortality. "Just as an aside, rupture can occur at any level of beta-HCG and whether beta-HCG is rising or falling or plateauing, so that doesn’t help you," she said.
More treatment options are available if ectopic pregnancy is diagnosed early, including methotrexate or conservative surgical treatment, and methotrexate is more efficacious in earlier than in later ectopic pregnancy, Dr. Jackson said.
Methotrexate treatment, which is relatively new for ectopic pregnancy, "is not for everyone," she added. "It involves a lot of follow-up. Patient compliance is incredibly important. There’s still 5% who will rupture despite methotrexate treatment. And all of these things need to be explained to the patient so she can make an informed choice." Methotrexate is less effective than salpingostomy, and the efficacy of the drug decreases with increasing beta-HCG levels. Fifteen percent of patients treated with methotrexate will require a second dose.
A decrease in future intrauterine pregnancy rates in women with ectopic pregnancy is no different in those treated with methotrexate than in those treated with surgery, and the risk of a future ectopic pregnancy is increased by 10%-15% in both groups, she said.
Dr. Jackson reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Make sure a woman who has uterine bleeding or abdominal pain in the first trimester of pregnancy gets an ultrasound the same day you see her, whether you do the ultrasound yourself, refer her to another physician for a same-day ultrasound, or send her to the emergency department, Dr. Rebecca Jackson advised.
The same-day ultrasound is essential to diagnose ectopic pregnancy early, Dr. Jackson said at a conference on women’s health sponsored by the University of California, San Francisco. The ultrasound is unlikely to show an ectopic pregnancy, but if you see an intrauterine pregnancy, "you’re done," she said.
Ectopic pregnancy occurs in 2% of pregnancies and is the leading cause of U.S. maternal deaths in the first trimester. "The key thing about ectopic pregnancy is that two-thirds of the women dying from an ectopic pregnancy had recently seen a clinician, but had an incorrect or delayed diagnosis," said Dr. Jackson, professor of obstetrics and gynecology at the university and chief of obstetrics and gynecology at San Francisco General Hospital.
If an ultrasound doesn’t show the location of the pregnancy, obtain serial quantitative beta-HCG levels to determine whether the pregnancy is normal or abnormal, she advised. "If it rises appropriately, it’s likely normal, but ectopics can also rise in the same way" as beta-HCG levels with intrauterine pregnancies, she said. "But if it drops or rises very little you know it’s abnormal."
Get a uterine aspiration in women with an abnormal beta-HCG level to look for placental tissue in the uterus, which indicates an intrauterine pregnancy, she said. If there’s no placental tissue in the uterus, treat for ectopic pregnancy.
There’s a shortcut in all of this to keep in mind, she added. If a woman presents with abnormal uterine bleeding or abdominal pain and she tests positive for pregnancy, ask if she desires the pregnancy. If not, you can skip the ultrasound and beta-HCG levels and go straight to the uterine aspiration, Dr. Jackson said.
These steps to diagnosing ectopic pregnancy may sound simple but in reality take ob.gyn. residents years to master. "There are so many flavors of how women present, and you combine that with their desires and their fertility desires, and it’s a really hard thing to manage," she said.
Only approximately 2% of ultrasounds done for possible ectopic pregnancy will show a gestational sac with a yolk sac or fetal pole visible outside the uterus, and thus be diagnostic, Dr. Jackson said. A normal adnexal exam does not exclude an ectopic pregnancy. An empty uterus on ultrasound and a beta-HCG level above the discriminatory zone suggests an ectopic pregnancy, and will be in 86% of cases. An ultrasound showing a complex mass and fluid in a cul-de-sac will be an ectopic pregnancy in 94% of cases. The main role of the ultrasound is to rule in an intrauterine pregnancy.
Diagnosing ectopic pregnancy early decreases the risk of rupture, which has been associated with decreased fertility and increased morbidity and mortality. "Just as an aside, rupture can occur at any level of beta-HCG and whether beta-HCG is rising or falling or plateauing, so that doesn’t help you," she said.
More treatment options are available if ectopic pregnancy is diagnosed early, including methotrexate or conservative surgical treatment, and methotrexate is more efficacious in earlier than in later ectopic pregnancy, Dr. Jackson said.
Methotrexate treatment, which is relatively new for ectopic pregnancy, "is not for everyone," she added. "It involves a lot of follow-up. Patient compliance is incredibly important. There’s still 5% who will rupture despite methotrexate treatment. And all of these things need to be explained to the patient so she can make an informed choice." Methotrexate is less effective than salpingostomy, and the efficacy of the drug decreases with increasing beta-HCG levels. Fifteen percent of patients treated with methotrexate will require a second dose.
A decrease in future intrauterine pregnancy rates in women with ectopic pregnancy is no different in those treated with methotrexate than in those treated with surgery, and the risk of a future ectopic pregnancy is increased by 10%-15% in both groups, she said.
Dr. Jackson reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Make sure a woman who has uterine bleeding or abdominal pain in the first trimester of pregnancy gets an ultrasound the same day you see her, whether you do the ultrasound yourself, refer her to another physician for a same-day ultrasound, or send her to the emergency department, Dr. Rebecca Jackson advised.
The same-day ultrasound is essential to diagnose ectopic pregnancy early, Dr. Jackson said at a conference on women’s health sponsored by the University of California, San Francisco. The ultrasound is unlikely to show an ectopic pregnancy, but if you see an intrauterine pregnancy, "you’re done," she said.
Ectopic pregnancy occurs in 2% of pregnancies and is the leading cause of U.S. maternal deaths in the first trimester. "The key thing about ectopic pregnancy is that two-thirds of the women dying from an ectopic pregnancy had recently seen a clinician, but had an incorrect or delayed diagnosis," said Dr. Jackson, professor of obstetrics and gynecology at the university and chief of obstetrics and gynecology at San Francisco General Hospital.
If an ultrasound doesn’t show the location of the pregnancy, obtain serial quantitative beta-HCG levels to determine whether the pregnancy is normal or abnormal, she advised. "If it rises appropriately, it’s likely normal, but ectopics can also rise in the same way" as beta-HCG levels with intrauterine pregnancies, she said. "But if it drops or rises very little you know it’s abnormal."
Get a uterine aspiration in women with an abnormal beta-HCG level to look for placental tissue in the uterus, which indicates an intrauterine pregnancy, she said. If there’s no placental tissue in the uterus, treat for ectopic pregnancy.
There’s a shortcut in all of this to keep in mind, she added. If a woman presents with abnormal uterine bleeding or abdominal pain and she tests positive for pregnancy, ask if she desires the pregnancy. If not, you can skip the ultrasound and beta-HCG levels and go straight to the uterine aspiration, Dr. Jackson said.
These steps to diagnosing ectopic pregnancy may sound simple but in reality take ob.gyn. residents years to master. "There are so many flavors of how women present, and you combine that with their desires and their fertility desires, and it’s a really hard thing to manage," she said.
Only approximately 2% of ultrasounds done for possible ectopic pregnancy will show a gestational sac with a yolk sac or fetal pole visible outside the uterus, and thus be diagnostic, Dr. Jackson said. A normal adnexal exam does not exclude an ectopic pregnancy. An empty uterus on ultrasound and a beta-HCG level above the discriminatory zone suggests an ectopic pregnancy, and will be in 86% of cases. An ultrasound showing a complex mass and fluid in a cul-de-sac will be an ectopic pregnancy in 94% of cases. The main role of the ultrasound is to rule in an intrauterine pregnancy.
Diagnosing ectopic pregnancy early decreases the risk of rupture, which has been associated with decreased fertility and increased morbidity and mortality. "Just as an aside, rupture can occur at any level of beta-HCG and whether beta-HCG is rising or falling or plateauing, so that doesn’t help you," she said.
More treatment options are available if ectopic pregnancy is diagnosed early, including methotrexate or conservative surgical treatment, and methotrexate is more efficacious in earlier than in later ectopic pregnancy, Dr. Jackson said.
Methotrexate treatment, which is relatively new for ectopic pregnancy, "is not for everyone," she added. "It involves a lot of follow-up. Patient compliance is incredibly important. There’s still 5% who will rupture despite methotrexate treatment. And all of these things need to be explained to the patient so she can make an informed choice." Methotrexate is less effective than salpingostomy, and the efficacy of the drug decreases with increasing beta-HCG levels. Fifteen percent of patients treated with methotrexate will require a second dose.
A decrease in future intrauterine pregnancy rates in women with ectopic pregnancy is no different in those treated with methotrexate than in those treated with surgery, and the risk of a future ectopic pregnancy is increased by 10%-15% in both groups, she said.
Dr. Jackson reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM A CONFERENCE ON WOMEN’S HEALTH
FDA investigating testosterone’s cardiovascular risks
The Food and Drug Administration is reassessing the cardiovascular safety of approved testosterone treatments and urged physicians and patients to report side effects.
Two recent observational studies that reported increased risks of MI, stroke, and death in men taking prescribed testosterone products prompted the FDA to open its investigation and to publish the alert about its actions.
Patients on prescribed testosterone products should not discontinue them without talking with their health care providers, the FDA cautioned. The agency has not concluded that approved testosterone formulations increase cardiovascular risk. Final recommendations are pending the completion of its evaluation.
The risk of MI doubled within 90 days of starting testosterone, compared with the previous year, in men aged 65 years or older and nearly tripled in younger men who had a prior history of heart disease, a cohort study of claims data for 55,593 men reported (PLoS ONE 2015 Jan. 29 [doi: 10.1371/journal.pone.0085805]).
Younger men with no history of heart disease showed no increased cardiovascular risk after filling a testosterone prescription, William D. Finkle and his associates reported. The National Cancer Institute funded the study. Mr. Finkle is owner of Consolidated Research, Los Angeles, a company that develops statistical methods and software. He and his associates reported having no relevant financial disclosures.
A separate retrospective cohort study of 8,709 men with low testosterone levels, 1,223 of whom were treated with testosterone, found a 30% increased risk for MI, stroke, or death within 1 year of starting testosterone compared with the men not on testosterone. The relative risk was threefold higher at 2 years and nearly sixfold higher at 3 years, reported Dr. Rebecca Vigen of the University of Texas at Southwestern Medical Center, Dallas, and her associates (JAMA 2013;310:1829-36).
The elevated risk with testosterone therapy was seen both in men who had existing coronary artery disease and in those who did not, and the risk did not appear to be affected by differences in cardiovascular risk factors such as blood pressure or by the use of preventive medications.
The Department of Veterans Affairs funded the study. Dr. Vigen and her associates reported having no relevant financial disclosures.
An earlier clinical trial of testosterone therapy in older men that included many men with cardiovascular disorders was halted early after an increase in cardiovascular events in the testosterone group compared with the placebo group was found, the National Institutes of Health reported in 2010.
The FDA urged health care professionals and patients to report side effects involving testosterone products to the FDA MedWatch program by filing a report online, calling 800-332-1088, or faxing to 800-FDA-0178.
FDA-approved testosterone products deliver the hormone by topical gel, transdermal patch, buccal system, and injection, and are indicated only for men with low or no testosterone levels associated with a medical condition. No testosterone products are approved to treat men with low testosterone levels that are not associated with a medical condition.
On Twitter @sherryboschert
The Food and Drug Administration is reassessing the cardiovascular safety of approved testosterone treatments and urged physicians and patients to report side effects.
Two recent observational studies that reported increased risks of MI, stroke, and death in men taking prescribed testosterone products prompted the FDA to open its investigation and to publish the alert about its actions.
Patients on prescribed testosterone products should not discontinue them without talking with their health care providers, the FDA cautioned. The agency has not concluded that approved testosterone formulations increase cardiovascular risk. Final recommendations are pending the completion of its evaluation.
The risk of MI doubled within 90 days of starting testosterone, compared with the previous year, in men aged 65 years or older and nearly tripled in younger men who had a prior history of heart disease, a cohort study of claims data for 55,593 men reported (PLoS ONE 2015 Jan. 29 [doi: 10.1371/journal.pone.0085805]).
Younger men with no history of heart disease showed no increased cardiovascular risk after filling a testosterone prescription, William D. Finkle and his associates reported. The National Cancer Institute funded the study. Mr. Finkle is owner of Consolidated Research, Los Angeles, a company that develops statistical methods and software. He and his associates reported having no relevant financial disclosures.
A separate retrospective cohort study of 8,709 men with low testosterone levels, 1,223 of whom were treated with testosterone, found a 30% increased risk for MI, stroke, or death within 1 year of starting testosterone compared with the men not on testosterone. The relative risk was threefold higher at 2 years and nearly sixfold higher at 3 years, reported Dr. Rebecca Vigen of the University of Texas at Southwestern Medical Center, Dallas, and her associates (JAMA 2013;310:1829-36).
The elevated risk with testosterone therapy was seen both in men who had existing coronary artery disease and in those who did not, and the risk did not appear to be affected by differences in cardiovascular risk factors such as blood pressure or by the use of preventive medications.
The Department of Veterans Affairs funded the study. Dr. Vigen and her associates reported having no relevant financial disclosures.
An earlier clinical trial of testosterone therapy in older men that included many men with cardiovascular disorders was halted early after an increase in cardiovascular events in the testosterone group compared with the placebo group was found, the National Institutes of Health reported in 2010.
The FDA urged health care professionals and patients to report side effects involving testosterone products to the FDA MedWatch program by filing a report online, calling 800-332-1088, or faxing to 800-FDA-0178.
FDA-approved testosterone products deliver the hormone by topical gel, transdermal patch, buccal system, and injection, and are indicated only for men with low or no testosterone levels associated with a medical condition. No testosterone products are approved to treat men with low testosterone levels that are not associated with a medical condition.
On Twitter @sherryboschert
The Food and Drug Administration is reassessing the cardiovascular safety of approved testosterone treatments and urged physicians and patients to report side effects.
Two recent observational studies that reported increased risks of MI, stroke, and death in men taking prescribed testosterone products prompted the FDA to open its investigation and to publish the alert about its actions.
Patients on prescribed testosterone products should not discontinue them without talking with their health care providers, the FDA cautioned. The agency has not concluded that approved testosterone formulations increase cardiovascular risk. Final recommendations are pending the completion of its evaluation.
The risk of MI doubled within 90 days of starting testosterone, compared with the previous year, in men aged 65 years or older and nearly tripled in younger men who had a prior history of heart disease, a cohort study of claims data for 55,593 men reported (PLoS ONE 2015 Jan. 29 [doi: 10.1371/journal.pone.0085805]).
Younger men with no history of heart disease showed no increased cardiovascular risk after filling a testosterone prescription, William D. Finkle and his associates reported. The National Cancer Institute funded the study. Mr. Finkle is owner of Consolidated Research, Los Angeles, a company that develops statistical methods and software. He and his associates reported having no relevant financial disclosures.
A separate retrospective cohort study of 8,709 men with low testosterone levels, 1,223 of whom were treated with testosterone, found a 30% increased risk for MI, stroke, or death within 1 year of starting testosterone compared with the men not on testosterone. The relative risk was threefold higher at 2 years and nearly sixfold higher at 3 years, reported Dr. Rebecca Vigen of the University of Texas at Southwestern Medical Center, Dallas, and her associates (JAMA 2013;310:1829-36).
The elevated risk with testosterone therapy was seen both in men who had existing coronary artery disease and in those who did not, and the risk did not appear to be affected by differences in cardiovascular risk factors such as blood pressure or by the use of preventive medications.
The Department of Veterans Affairs funded the study. Dr. Vigen and her associates reported having no relevant financial disclosures.
An earlier clinical trial of testosterone therapy in older men that included many men with cardiovascular disorders was halted early after an increase in cardiovascular events in the testosterone group compared with the placebo group was found, the National Institutes of Health reported in 2010.
The FDA urged health care professionals and patients to report side effects involving testosterone products to the FDA MedWatch program by filing a report online, calling 800-332-1088, or faxing to 800-FDA-0178.
FDA-approved testosterone products deliver the hormone by topical gel, transdermal patch, buccal system, and injection, and are indicated only for men with low or no testosterone levels associated with a medical condition. No testosterone products are approved to treat men with low testosterone levels that are not associated with a medical condition.
On Twitter @sherryboschert