User login
Bilateral Auricular Swelling: Marginal Zone Lymphoma With Cutaneous Involvement
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.
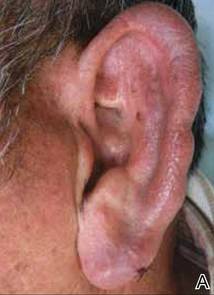 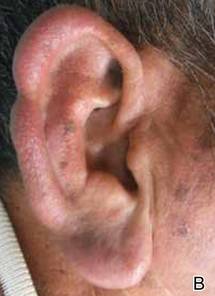 |
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
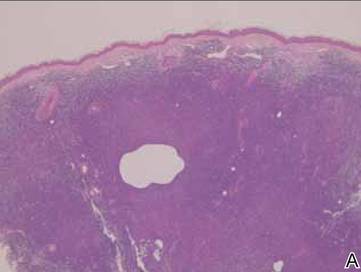 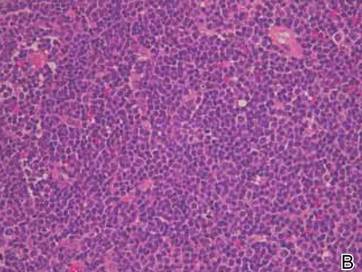 Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.
  |
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
  Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.
  |
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
  Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
