User login
Betamethasone Dipropionate Spray 0.05% Alleviates Troublesome Symptoms of Plaque Psoriasis
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
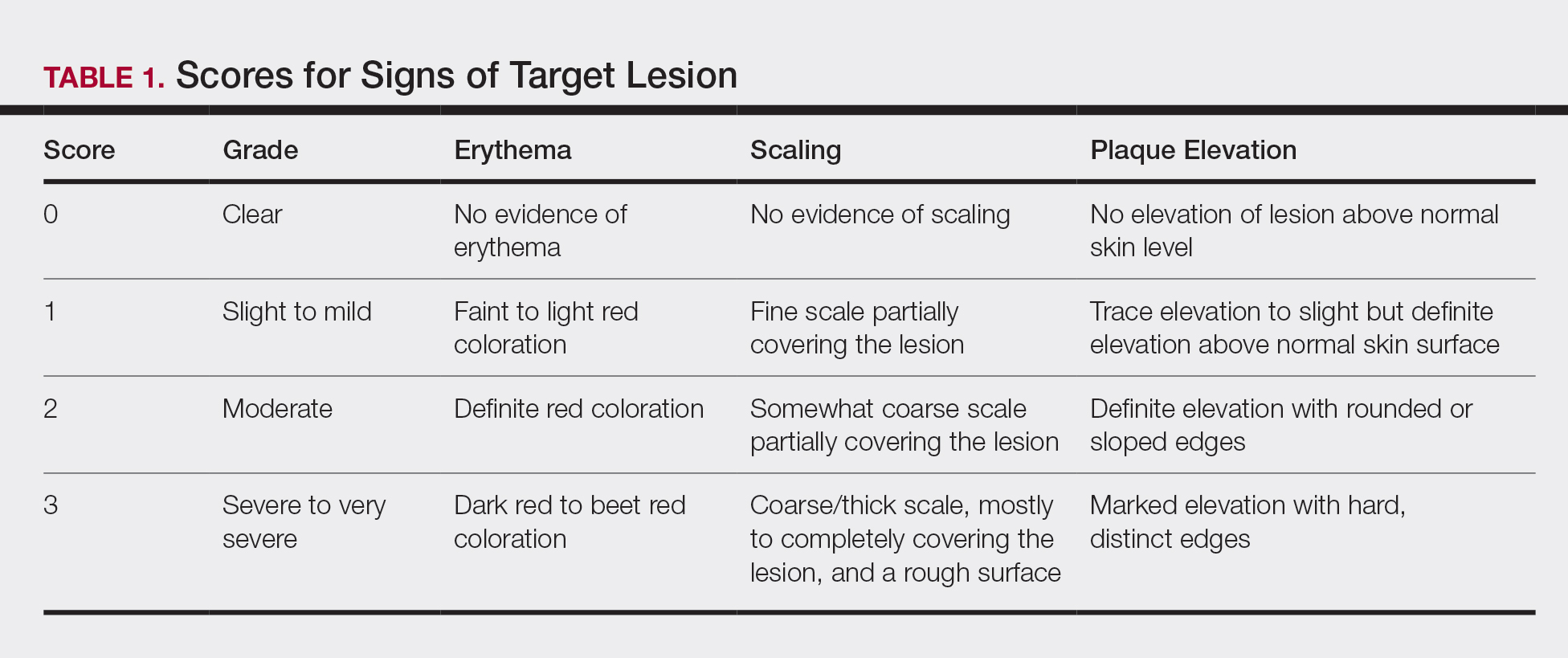
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
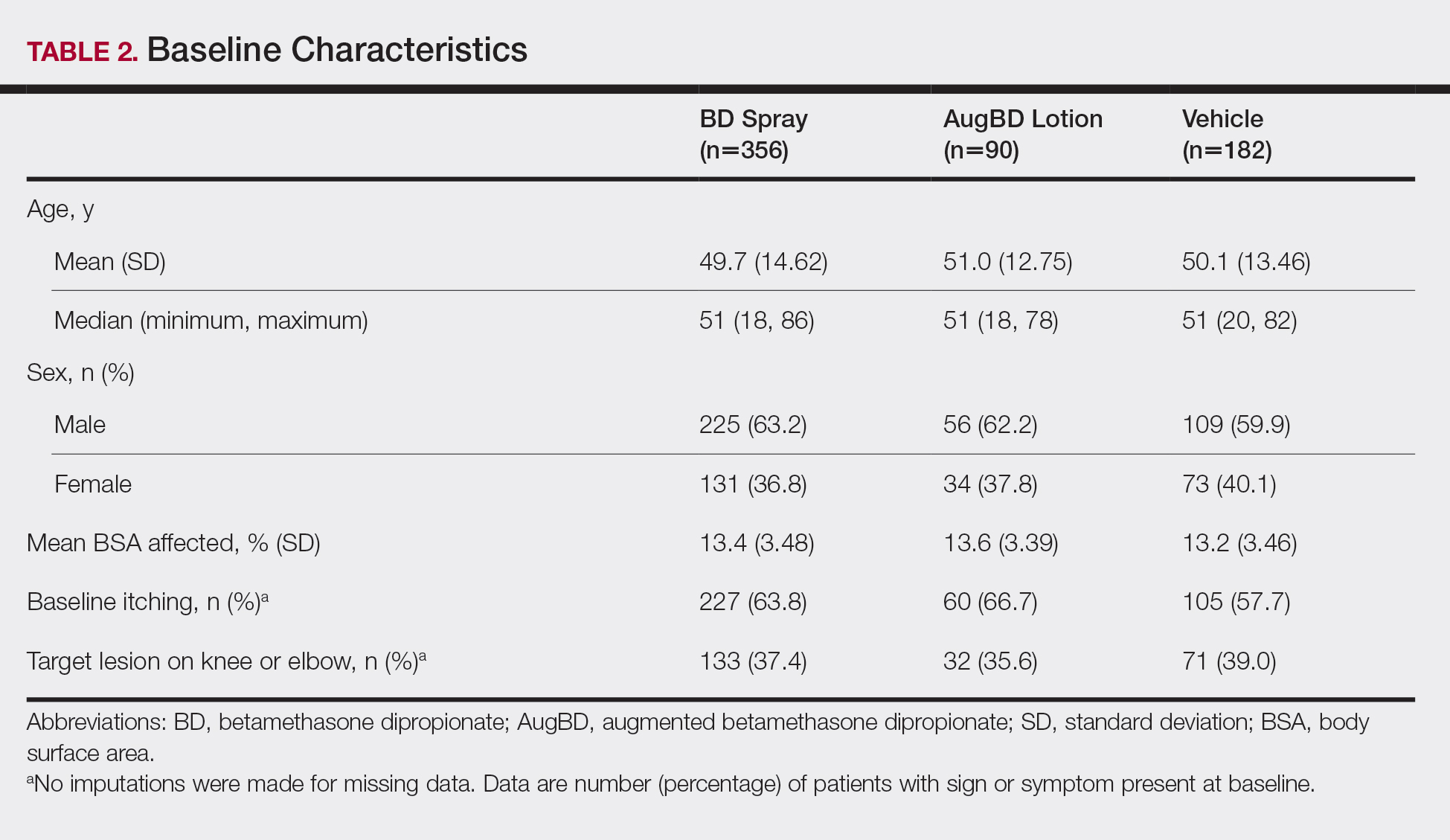
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
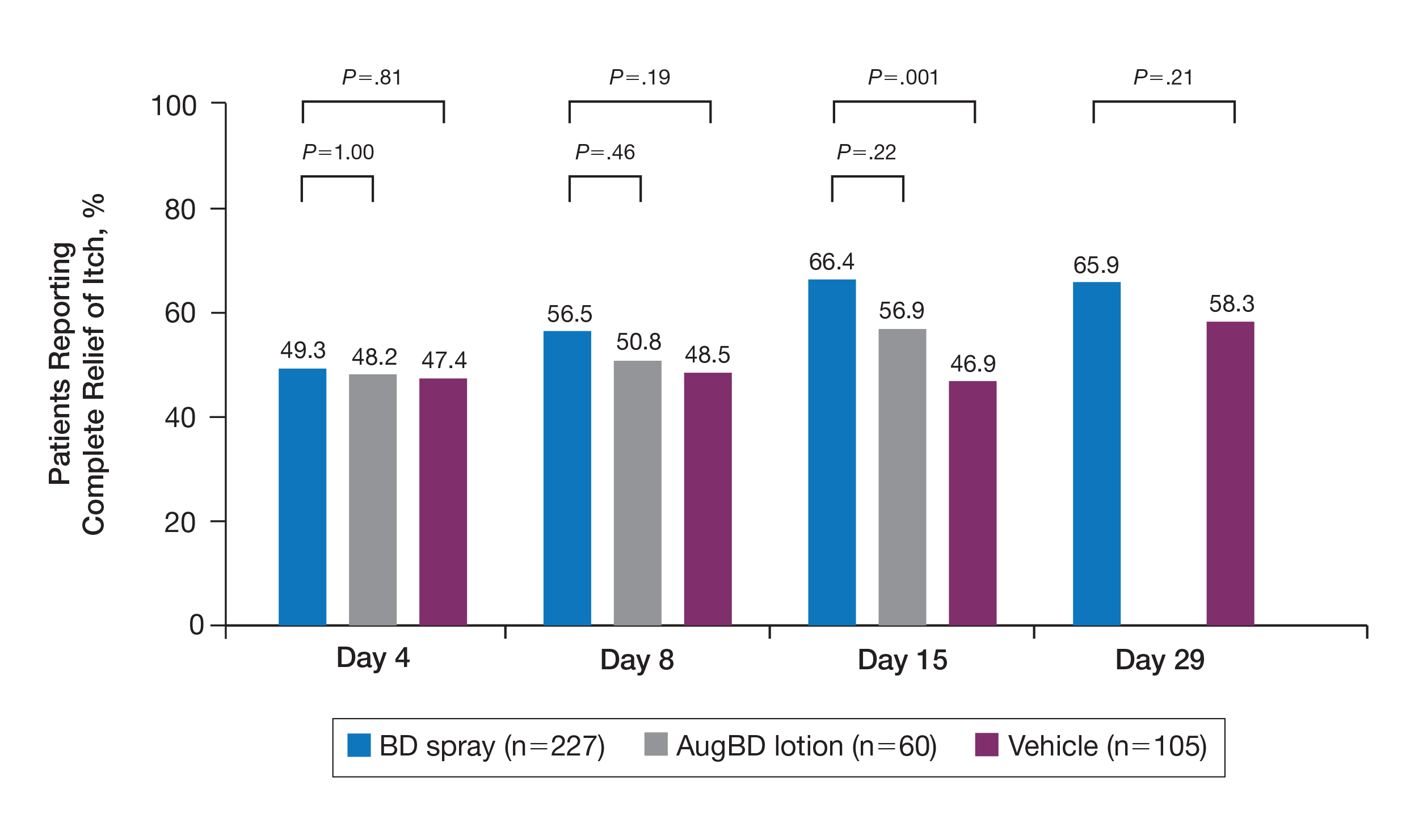
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
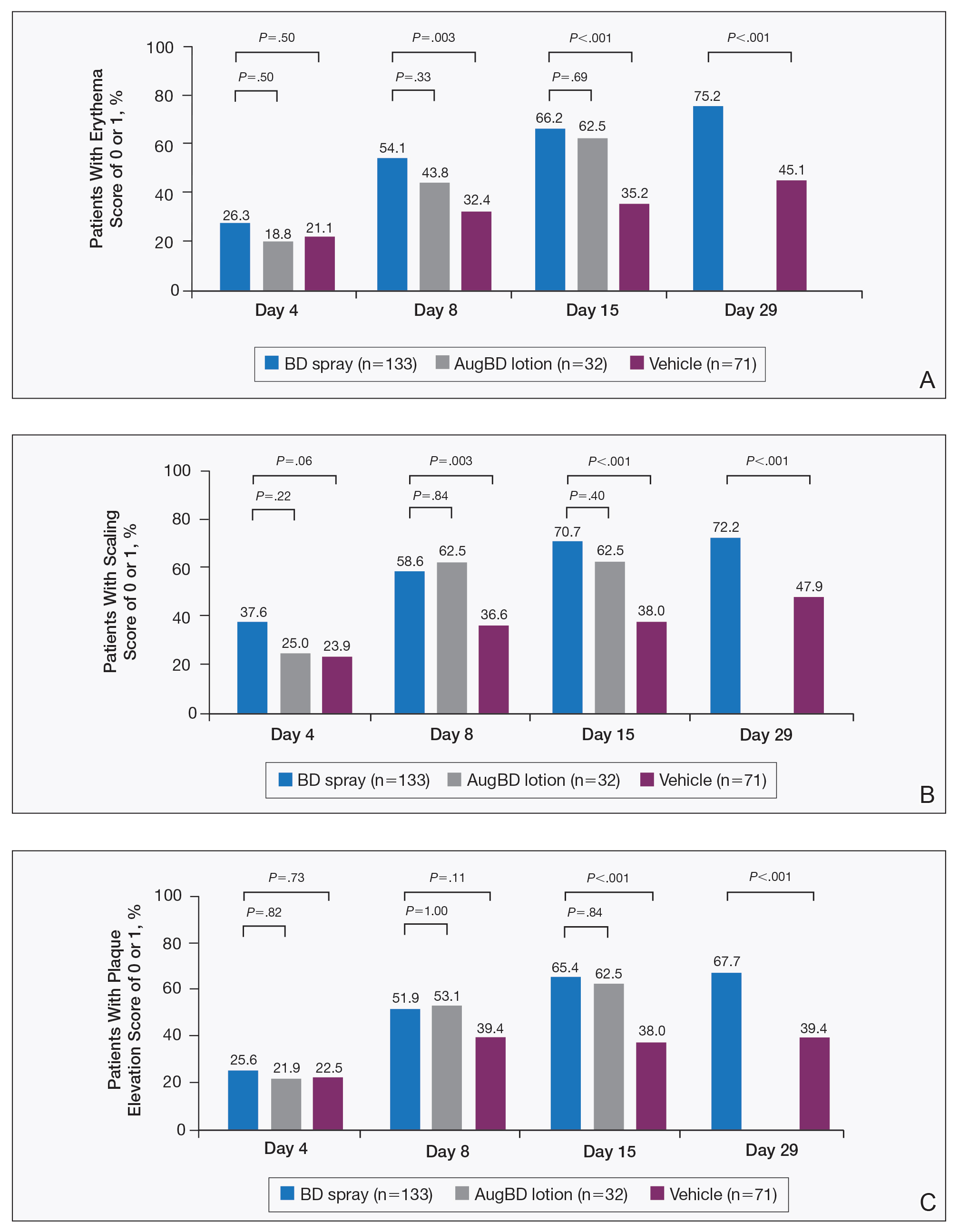
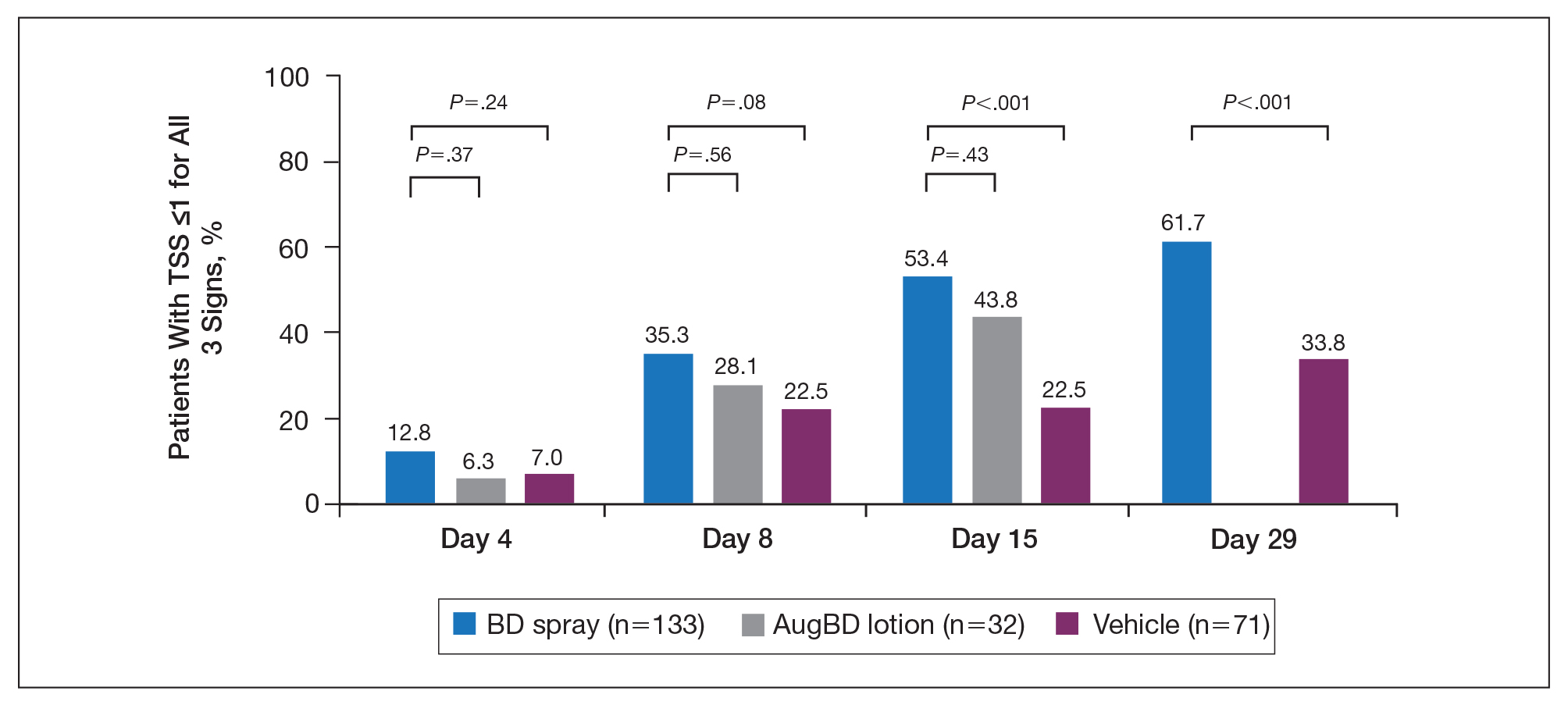
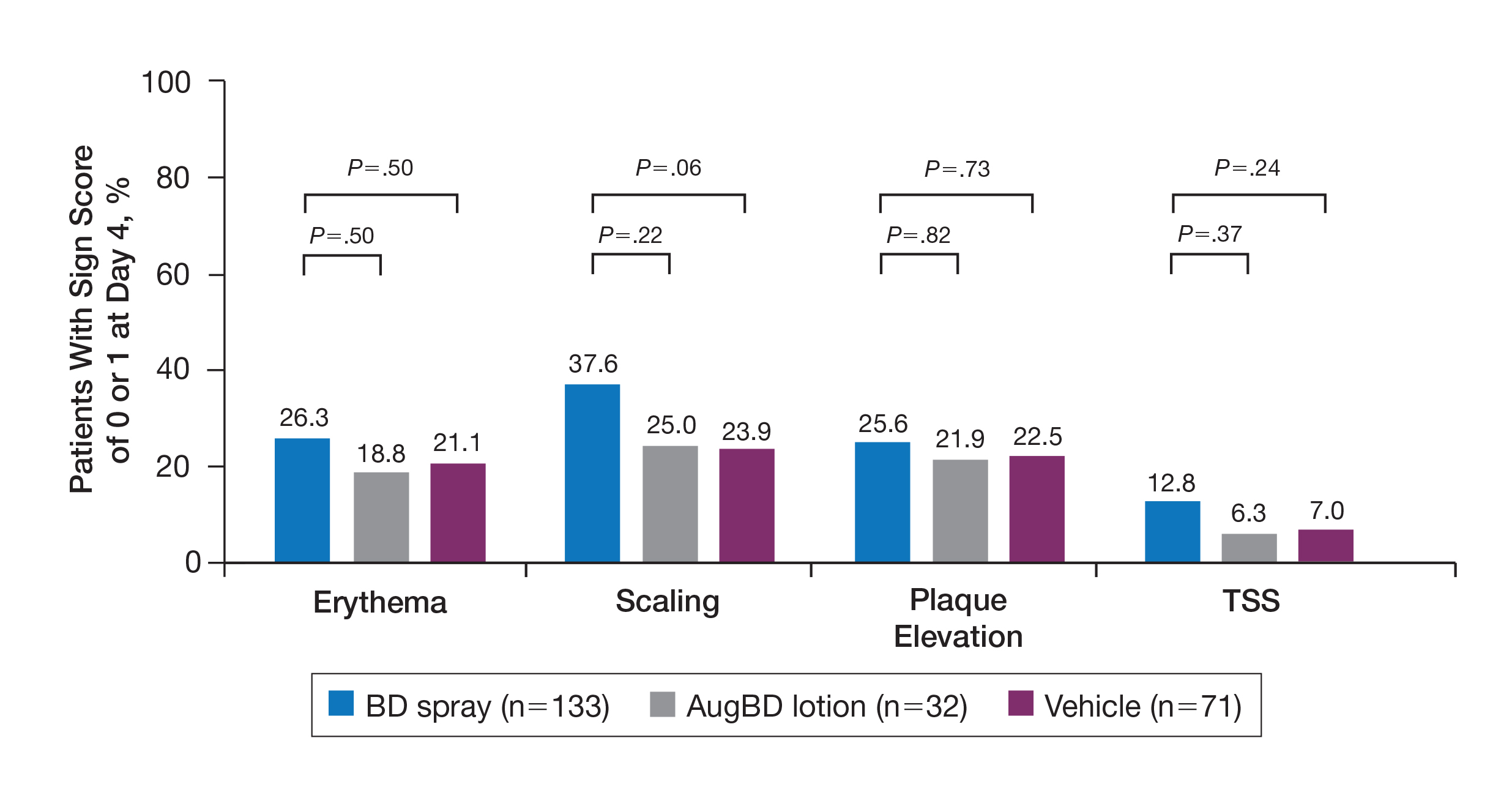
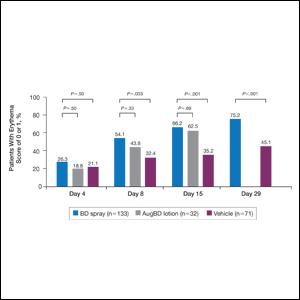
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Practice Points
- Pruritus is one of the most bothersome symptoms of psoriasis; plaques located on the knees and elbows remain hard to treat.
- Topical corticosteroids are the initial form of treatment of localized plaque psoriasis.
- The choice of vehicle can change the penetration of the medication, alter the efficacy, and minimize side effects of the drug.
- Betamethasone dipropionate spray 0.05% is a mid-potent corticosteroid that provides fast symptom relief and early efficacy in clearing plaques, similar to a high-potency topical corticosteroid but with less potential for systemic absorption and adverse events.