User login
No pain, if you’ve got game
ILLUSTRATIVE CASE
An 8-year-old girl with congenital heart disease (status: post repair) arrives at your clinic for a routine appointment. Since the age of 12 months, she has experienced significant anxiety during medical visits, especially with blood draws and injections. She enjoys playing video games on her new tablet computer. Her parents want to know what you can do to reduce her anxiety and pain during today’s scheduled blood draw. Should you recommend that she continue playing video games during the venipuncture?
Adequately managing pain while performing venipuncture in children can improve the quality of the experience, reduce children’s fear of going to the doctor, and increase efficiency in medical practice.2 Since pharmacologic pain-control methods may have adverse effects, distraction techniques—engaging the child in another activity during a procedure—are commonly used instead to help reduce a child’s pain. These techniques can be active or passive.
Studies have demonstrated that both active and passive distraction techniques reduce children’s pain during medical procedures, including venipuncture. Passive techniques, such as nurse coaching3 and watching cartoons,4 have been found to reduce distress and pain. Active distraction techniques, such as playing video games while undergoing a painful procedure (eg, dressing a wound), have been shown to be more effective than passive techniques.5,6
A Cochrane review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children demonstrated reduced pain, but the quality of evidence was low and the review recommended improved methodological rigor and trial reporting.7 Another systematic review and analysis showed strong support for distraction for reducing pain; however, the quality of evidence was low and the researchers cited problems with characteristics of the distraction interventions, child age, and risk of bias in the studies.8
There has been a lack of RCTs comparing the effectiveness and superiority of active vs passive distraction techniques. The first high-quality RCT to directly compare 3 of the most common distraction techniques to a control group was recently conducted in a large training and research hospital in Turkey.1
STUDY SUMMARY
Pain and anxiety levels were lowest in actively distracted children
The RCT included 180 children ages 6 to 10 years randomly assigned to 1 of 3 intervention groups or a control group.1 Phlebotomy was performed while children watched a cartoon, played a video game, were distracted by parental interaction, or had no distraction (control group).
Investigators independently measured pain and anxiety in the patient and perceived pain and anxiety according to both a family member and a health care worker (medical observer). Researchers used the previously validated Children’s Fear Scale and the Wong-Baker Pain Scale.9,10 The Children’s Fear Scale was used to assess anxiety in children on a scale of 0 (picture of a calm face) to 4 (picture of the most fearful face). The Wong-Baker Pain Scale was used to assess pain on a scale of 0 (no hurt: happy face) to 10 (hurts worst: saddest face).
Continue to: Results
Results. The pain and anxiety scores were significantly lower in all of the intervention groups compared with the control group (P < .05). The video game (active distraction) group had the lowest levels of both pain and anxiety. The self-reported Children’s Fear Scale scores of children in the video game group were 0.27, compared with 0.76 in the cartoon group, 1.24 in the parental distraction group, and 2.22 in the control group. The anxiety scores recorded by the family member and the medical observer showed similar significant differences.
The Wong-Baker Pain Scale scores showed similar differences in self-reported pain for the video game group (1.42) compared with the cartoon group (3.02), the parental distraction group (2.89), and the control group (5.11). Pain scores reported by the family member and the medical observer (respectively) also reflected benefit from any type of distraction, with active game-playing as the most effective type of distraction (video game: 1.69 and 1.96; cartoon: 3.07 and 3.20; parental distraction: 3.56 and 4.22; and control: 5.29 and 6.13).
In addition, the intraclass correlation coefficient was 0.67 to 0.924 (P < .01), suggesting that the reports from the child, parent, and medical observer about the child’s pain and anxiety were highly correlated.
WHAT'S NEW
All distraction techniques provide benefit, but there’s a clear winner
In this RCT of children undergoing phlebotomy, both active and passive distraction techniques were superior to no distraction in terms of perceived pain and anxiety by the child, a health care provider, or a parent. The active-distraction group played a video game, while the passive-distraction groups watched a cartoon or interacted with a parent. Active distraction was superior to passive distraction.
CAVEATS
Procedure time was short; intervention not blinded
One potential weakness of this study is that it was not a double-blinded trial. Blinding was not possible for much of the study as the patient, parent, and medical observer were fully aware of the intervention or lack thereof. However, the parent and medical observer were blinded to each other’s assessments of the child’s pain and anxiety.
Continue to: Furthermore, the study...
Furthermore, the study was conducted at a single institution in Turkey. There could be cultural differences in reporting of pain and anxiety compared to Western cultures.
Finally, the average duration of the procedure in this study was 3 minutes, with a range of 1 to 5 minutes. It is unclear if the findings can be extrapolated to more time-consuming procedures.
CHALLENGES TO IMPLEMENTATION
Technology is not available to all
The use of tablet computers may seem increasingly ubiquitous, but not all families have access to these devices. Another challenge is that phlebotomy/clinic personnel must learn to work around the device.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Inan G, Inal S. The impact of 3 different distraction techniques on the pain and anxiety levels of children during venipuncture: a clinical trial. Clin J Pain. 2019;35:140-147.
2. Fein JA, Zempsky WT, Cravero JP, Committee on Pediatric Emergency Medicine and Section on Anesthesiology and Pain Medicine; American Academy of Pediatrics. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2012;130:e1391-e1405.
3. Cohen LL, Blount RL, Panopoulos G. Nurse coaching and cartoon distraction: an effective and practical intervention to reduce child, parent, and nurse distress during immunizations. J Pediatr Psychol. 1997;22:355-370.
4. Downey VA, Zun LS. The impact of watching cartoons for distraction during painful procedures in the emergency department. Pediatr Emerg. 2012;28:1033-1035.
5. Hussein H. Effect of active and passive distraction on decreasing pain associated with painful medical procedures among school aged children. World J Nurs Sci. 2015;1:13-23.
6. Nilsson S, Enskär K, Hallqvist C, et al. Active and passive distraction in children undergoing wound dressing. J Pediatr Nurs. 2013;28:158-166.
7. Birnie KA, Noel M, Chambers CT, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018;10:CD005179.
8. Birnie KA, Noel M, Parker JA, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psychol. 2014;39:783-808.
9. McMurtry CM, Noel M, Chambers CT, et al. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30:780-788.
10. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatric Nurs. 1988;14:9-17.
ILLUSTRATIVE CASE
An 8-year-old girl with congenital heart disease (status: post repair) arrives at your clinic for a routine appointment. Since the age of 12 months, she has experienced significant anxiety during medical visits, especially with blood draws and injections. She enjoys playing video games on her new tablet computer. Her parents want to know what you can do to reduce her anxiety and pain during today’s scheduled blood draw. Should you recommend that she continue playing video games during the venipuncture?
Adequately managing pain while performing venipuncture in children can improve the quality of the experience, reduce children’s fear of going to the doctor, and increase efficiency in medical practice.2 Since pharmacologic pain-control methods may have adverse effects, distraction techniques—engaging the child in another activity during a procedure—are commonly used instead to help reduce a child’s pain. These techniques can be active or passive.
Studies have demonstrated that both active and passive distraction techniques reduce children’s pain during medical procedures, including venipuncture. Passive techniques, such as nurse coaching3 and watching cartoons,4 have been found to reduce distress and pain. Active distraction techniques, such as playing video games while undergoing a painful procedure (eg, dressing a wound), have been shown to be more effective than passive techniques.5,6
A Cochrane review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children demonstrated reduced pain, but the quality of evidence was low and the review recommended improved methodological rigor and trial reporting.7 Another systematic review and analysis showed strong support for distraction for reducing pain; however, the quality of evidence was low and the researchers cited problems with characteristics of the distraction interventions, child age, and risk of bias in the studies.8
There has been a lack of RCTs comparing the effectiveness and superiority of active vs passive distraction techniques. The first high-quality RCT to directly compare 3 of the most common distraction techniques to a control group was recently conducted in a large training and research hospital in Turkey.1
STUDY SUMMARY
Pain and anxiety levels were lowest in actively distracted children
The RCT included 180 children ages 6 to 10 years randomly assigned to 1 of 3 intervention groups or a control group.1 Phlebotomy was performed while children watched a cartoon, played a video game, were distracted by parental interaction, or had no distraction (control group).
Investigators independently measured pain and anxiety in the patient and perceived pain and anxiety according to both a family member and a health care worker (medical observer). Researchers used the previously validated Children’s Fear Scale and the Wong-Baker Pain Scale.9,10 The Children’s Fear Scale was used to assess anxiety in children on a scale of 0 (picture of a calm face) to 4 (picture of the most fearful face). The Wong-Baker Pain Scale was used to assess pain on a scale of 0 (no hurt: happy face) to 10 (hurts worst: saddest face).
Continue to: Results
Results. The pain and anxiety scores were significantly lower in all of the intervention groups compared with the control group (P < .05). The video game (active distraction) group had the lowest levels of both pain and anxiety. The self-reported Children’s Fear Scale scores of children in the video game group were 0.27, compared with 0.76 in the cartoon group, 1.24 in the parental distraction group, and 2.22 in the control group. The anxiety scores recorded by the family member and the medical observer showed similar significant differences.
The Wong-Baker Pain Scale scores showed similar differences in self-reported pain for the video game group (1.42) compared with the cartoon group (3.02), the parental distraction group (2.89), and the control group (5.11). Pain scores reported by the family member and the medical observer (respectively) also reflected benefit from any type of distraction, with active game-playing as the most effective type of distraction (video game: 1.69 and 1.96; cartoon: 3.07 and 3.20; parental distraction: 3.56 and 4.22; and control: 5.29 and 6.13).
In addition, the intraclass correlation coefficient was 0.67 to 0.924 (P < .01), suggesting that the reports from the child, parent, and medical observer about the child’s pain and anxiety were highly correlated.
WHAT'S NEW
All distraction techniques provide benefit, but there’s a clear winner
In this RCT of children undergoing phlebotomy, both active and passive distraction techniques were superior to no distraction in terms of perceived pain and anxiety by the child, a health care provider, or a parent. The active-distraction group played a video game, while the passive-distraction groups watched a cartoon or interacted with a parent. Active distraction was superior to passive distraction.
CAVEATS
Procedure time was short; intervention not blinded
One potential weakness of this study is that it was not a double-blinded trial. Blinding was not possible for much of the study as the patient, parent, and medical observer were fully aware of the intervention or lack thereof. However, the parent and medical observer were blinded to each other’s assessments of the child’s pain and anxiety.
Continue to: Furthermore, the study...
Furthermore, the study was conducted at a single institution in Turkey. There could be cultural differences in reporting of pain and anxiety compared to Western cultures.
Finally, the average duration of the procedure in this study was 3 minutes, with a range of 1 to 5 minutes. It is unclear if the findings can be extrapolated to more time-consuming procedures.
CHALLENGES TO IMPLEMENTATION
Technology is not available to all
The use of tablet computers may seem increasingly ubiquitous, but not all families have access to these devices. Another challenge is that phlebotomy/clinic personnel must learn to work around the device.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
An 8-year-old girl with congenital heart disease (status: post repair) arrives at your clinic for a routine appointment. Since the age of 12 months, she has experienced significant anxiety during medical visits, especially with blood draws and injections. She enjoys playing video games on her new tablet computer. Her parents want to know what you can do to reduce her anxiety and pain during today’s scheduled blood draw. Should you recommend that she continue playing video games during the venipuncture?
Adequately managing pain while performing venipuncture in children can improve the quality of the experience, reduce children’s fear of going to the doctor, and increase efficiency in medical practice.2 Since pharmacologic pain-control methods may have adverse effects, distraction techniques—engaging the child in another activity during a procedure—are commonly used instead to help reduce a child’s pain. These techniques can be active or passive.
Studies have demonstrated that both active and passive distraction techniques reduce children’s pain during medical procedures, including venipuncture. Passive techniques, such as nurse coaching3 and watching cartoons,4 have been found to reduce distress and pain. Active distraction techniques, such as playing video games while undergoing a painful procedure (eg, dressing a wound), have been shown to be more effective than passive techniques.5,6
A Cochrane review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children demonstrated reduced pain, but the quality of evidence was low and the review recommended improved methodological rigor and trial reporting.7 Another systematic review and analysis showed strong support for distraction for reducing pain; however, the quality of evidence was low and the researchers cited problems with characteristics of the distraction interventions, child age, and risk of bias in the studies.8
There has been a lack of RCTs comparing the effectiveness and superiority of active vs passive distraction techniques. The first high-quality RCT to directly compare 3 of the most common distraction techniques to a control group was recently conducted in a large training and research hospital in Turkey.1
STUDY SUMMARY
Pain and anxiety levels were lowest in actively distracted children
The RCT included 180 children ages 6 to 10 years randomly assigned to 1 of 3 intervention groups or a control group.1 Phlebotomy was performed while children watched a cartoon, played a video game, were distracted by parental interaction, or had no distraction (control group).
Investigators independently measured pain and anxiety in the patient and perceived pain and anxiety according to both a family member and a health care worker (medical observer). Researchers used the previously validated Children’s Fear Scale and the Wong-Baker Pain Scale.9,10 The Children’s Fear Scale was used to assess anxiety in children on a scale of 0 (picture of a calm face) to 4 (picture of the most fearful face). The Wong-Baker Pain Scale was used to assess pain on a scale of 0 (no hurt: happy face) to 10 (hurts worst: saddest face).
Continue to: Results
Results. The pain and anxiety scores were significantly lower in all of the intervention groups compared with the control group (P < .05). The video game (active distraction) group had the lowest levels of both pain and anxiety. The self-reported Children’s Fear Scale scores of children in the video game group were 0.27, compared with 0.76 in the cartoon group, 1.24 in the parental distraction group, and 2.22 in the control group. The anxiety scores recorded by the family member and the medical observer showed similar significant differences.
The Wong-Baker Pain Scale scores showed similar differences in self-reported pain for the video game group (1.42) compared with the cartoon group (3.02), the parental distraction group (2.89), and the control group (5.11). Pain scores reported by the family member and the medical observer (respectively) also reflected benefit from any type of distraction, with active game-playing as the most effective type of distraction (video game: 1.69 and 1.96; cartoon: 3.07 and 3.20; parental distraction: 3.56 and 4.22; and control: 5.29 and 6.13).
In addition, the intraclass correlation coefficient was 0.67 to 0.924 (P < .01), suggesting that the reports from the child, parent, and medical observer about the child’s pain and anxiety were highly correlated.
WHAT'S NEW
All distraction techniques provide benefit, but there’s a clear winner
In this RCT of children undergoing phlebotomy, both active and passive distraction techniques were superior to no distraction in terms of perceived pain and anxiety by the child, a health care provider, or a parent. The active-distraction group played a video game, while the passive-distraction groups watched a cartoon or interacted with a parent. Active distraction was superior to passive distraction.
CAVEATS
Procedure time was short; intervention not blinded
One potential weakness of this study is that it was not a double-blinded trial. Blinding was not possible for much of the study as the patient, parent, and medical observer were fully aware of the intervention or lack thereof. However, the parent and medical observer were blinded to each other’s assessments of the child’s pain and anxiety.
Continue to: Furthermore, the study...
Furthermore, the study was conducted at a single institution in Turkey. There could be cultural differences in reporting of pain and anxiety compared to Western cultures.
Finally, the average duration of the procedure in this study was 3 minutes, with a range of 1 to 5 minutes. It is unclear if the findings can be extrapolated to more time-consuming procedures.
CHALLENGES TO IMPLEMENTATION
Technology is not available to all
The use of tablet computers may seem increasingly ubiquitous, but not all families have access to these devices. Another challenge is that phlebotomy/clinic personnel must learn to work around the device.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Inan G, Inal S. The impact of 3 different distraction techniques on the pain and anxiety levels of children during venipuncture: a clinical trial. Clin J Pain. 2019;35:140-147.
2. Fein JA, Zempsky WT, Cravero JP, Committee on Pediatric Emergency Medicine and Section on Anesthesiology and Pain Medicine; American Academy of Pediatrics. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2012;130:e1391-e1405.
3. Cohen LL, Blount RL, Panopoulos G. Nurse coaching and cartoon distraction: an effective and practical intervention to reduce child, parent, and nurse distress during immunizations. J Pediatr Psychol. 1997;22:355-370.
4. Downey VA, Zun LS. The impact of watching cartoons for distraction during painful procedures in the emergency department. Pediatr Emerg. 2012;28:1033-1035.
5. Hussein H. Effect of active and passive distraction on decreasing pain associated with painful medical procedures among school aged children. World J Nurs Sci. 2015;1:13-23.
6. Nilsson S, Enskär K, Hallqvist C, et al. Active and passive distraction in children undergoing wound dressing. J Pediatr Nurs. 2013;28:158-166.
7. Birnie KA, Noel M, Chambers CT, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018;10:CD005179.
8. Birnie KA, Noel M, Parker JA, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psychol. 2014;39:783-808.
9. McMurtry CM, Noel M, Chambers CT, et al. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30:780-788.
10. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatric Nurs. 1988;14:9-17.
1. Inan G, Inal S. The impact of 3 different distraction techniques on the pain and anxiety levels of children during venipuncture: a clinical trial. Clin J Pain. 2019;35:140-147.
2. Fein JA, Zempsky WT, Cravero JP, Committee on Pediatric Emergency Medicine and Section on Anesthesiology and Pain Medicine; American Academy of Pediatrics. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2012;130:e1391-e1405.
3. Cohen LL, Blount RL, Panopoulos G. Nurse coaching and cartoon distraction: an effective and practical intervention to reduce child, parent, and nurse distress during immunizations. J Pediatr Psychol. 1997;22:355-370.
4. Downey VA, Zun LS. The impact of watching cartoons for distraction during painful procedures in the emergency department. Pediatr Emerg. 2012;28:1033-1035.
5. Hussein H. Effect of active and passive distraction on decreasing pain associated with painful medical procedures among school aged children. World J Nurs Sci. 2015;1:13-23.
6. Nilsson S, Enskär K, Hallqvist C, et al. Active and passive distraction in children undergoing wound dressing. J Pediatr Nurs. 2013;28:158-166.
7. Birnie KA, Noel M, Chambers CT, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018;10:CD005179.
8. Birnie KA, Noel M, Parker JA, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psychol. 2014;39:783-808.
9. McMurtry CM, Noel M, Chambers CT, et al. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30:780-788.
10. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatric Nurs. 1988;14:9-17.
PRACTICE CHANGER
Employ active distraction, such as playing a video game, rather than passive distraction (eg, watching a video) to reduce pain and anxiety during pediatric venipuncture.
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, randomized controlled trial (RCT). 1
Inan G, Inal S. The impact of 3 different distraction techniques on the pain and anxiety levels of children during venipuncture: a clinical trial. Clin J Pain. 2019;35:140-147.
How can you prevent migraines during pregnancy?
No randomized controlled trials (RCT) have addressed pharmacologic prophylaxis of migraine for pregnant women. Two studies suggest that nonpharmacologic therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) not only relieved acute pain, but also decreased the frequency of headaches (strength of recommendation [SOR]: B, poor-quality cohort and RCTs).
Practice guidelines and most review articles recommend avoiding prophylactic medications if possible. If a medication must be used, base the selection on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies (SOR: C, expert opinion).
Nonpharmacologic approaches, limited analgesics remain the mainstay of prevention for pregnant women
Robert Sheeler, MD
Mayo Clinic, Rochester, Minn
Standards of care favor minimizing any use of drugs with known or even theoretical risk to the fetus. That includes virtually all the classes of drugs prescribed for migraine prevention. Limited use of analgesics such as acetaminophen and opioids for migraine-abortive treatment is closer to the standard of care for severe headaches. The nonpharmacologic approaches delineated in this article are the mainstay of treatment.
Prophylactic pharmacotherapy for migraine would be more justifiable if it also treated other conditions in which the risks/benefits for both the mother and fetus were clearer, such as maternal hypertension (in which labetalol can be effective for both conditions), or severe depression. Other nontraditional therapies that have shown efficacy for nonpregnant patients such as magnesium supplementation may be worthy of study since the risk of fetal harm in all trimesters appears remote.
In all cases, carefully documenting patient involvement in risk/benefit discussions.
Evidence summary
Eighteen percent of all women report migraines.1 Among pregnant migraineurs, 2.5% to 8% reported worsening symptoms.1,2 Guidelines recommend considering prophylaxis for nonpregnant patients if they experience at least 3 or 4 prolonged severe attacks per month.3
Nonpharmacological treatment. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed alleviation of symptoms for 15 of 19 women. The second, a small unblinded RCT, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. Over 72% of the treatment arm improved compared with nearly 29% of the control group.4 The 30 women (19 from the original cohort and the 11 from the intervention arm of the RCT) were then followed as a cohort for the duration of pregnancy and 1 year postpartum. More than 67% of the patients continued to report a decrease in the frequency and severity of headache.5 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Pharmacologic agents. Randomized controlled trials have demonstrated that multiple medications have prophylactic benefit in the treatment of nonpregnant patients with migraine. In particular, propanolol,6 divalproex sodium/sodium valproate, and topiramate7 have been effective. A single case report on the use of labetalol by a pregnant woman at 28 weeks’ gestational age showed that it was effective in reducing the frequency and severity of her headaches after 1 week of use. This improvement persisted until delivery at 38 weeks.8
Safety in pregnancy. The Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.9 There are no data about the effectiveness of medications for migraine prophylaxis in pregnancy so one cannot select a specific medication with certainty. However, it may be reasonable to select medications based on both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary.
The TABLE shows commonly used drugs for prophylaxis of migraine and their pregnancy risk category classification. It should be noted that even if risk has been demonstrated in a medication, not all risks are equal. For example, propanolol is class D because of increased risk for intrauterine growth restriction in the third trimester, while sodium valproate is class D because of known teratogenicity.9
TABLE
Pregnancy risk category of some prophylactic drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Labetalol (Normodyne, Trandate) | C/D* |
| Propanolol (Inderal, Inderide) | C/D* |
| Verapamil (Calan, Isoptin, Verelan) | C |
| Nifedipine (Procardia) | C |
| Amitriptyline (Limbitrol) | C |
| Nortriptyline (Aventyl, Pamelor) | D |
| Fluoxetine (Prozac) | C |
| Gabapentin (Neurontin) | C |
| Divalproex sodium (Depakote) | D |
| Topiramate (Topama x) | C |
| A=controlled human studies show no risk, B=No evidence of risk in humans, but no controlled studies, C=Risk to humans has not been rule d out, D=Positive evidence of risk to humans from human or animal studies, X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.9 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend avoidance of prophylactic medications in pregnancy, if possible. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy. If drug treatment is necessary, they recommend selecting an agent with the lowest risk of adverse effects to the fetus.3 Most review articles state that, if medication is necessary, it should be tailored towards other comorbidities, if possible; if there are no coexisting conditions, then calcium channel blockers or beta blockers would be the treatment of choice, based on safety data.1,10
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 2004;22:727-756.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence base d review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Scharff L, Marcus DA, Turk DC. Maintenance of effects in the nonmedical treatment of headaches during pregnancy. Headache 1996;36:285-290
6. Linde K, Rossnagel K. Propanolol for migraine prophylaxis. Cochrane Database Syst Rev 2004;(2):CD003225-
7. Chronicle E, Mulleners W. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev 2004;(3):CD003226-
8. Dey R, Khan S, Akhouri V, Wootton J, Bajwa Z. Labetalol for prophylactic treatment of intractable migraine during pregnancy. Headache 2002;42:642-645.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins, 2002.
10. Martin SR, Foley MR. Approach to the pregnant patient with headache. Clin Obstet Gynecol 2005;48:2-11.
No randomized controlled trials (RCT) have addressed pharmacologic prophylaxis of migraine for pregnant women. Two studies suggest that nonpharmacologic therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) not only relieved acute pain, but also decreased the frequency of headaches (strength of recommendation [SOR]: B, poor-quality cohort and RCTs).
Practice guidelines and most review articles recommend avoiding prophylactic medications if possible. If a medication must be used, base the selection on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies (SOR: C, expert opinion).
Nonpharmacologic approaches, limited analgesics remain the mainstay of prevention for pregnant women
Robert Sheeler, MD
Mayo Clinic, Rochester, Minn
Standards of care favor minimizing any use of drugs with known or even theoretical risk to the fetus. That includes virtually all the classes of drugs prescribed for migraine prevention. Limited use of analgesics such as acetaminophen and opioids for migraine-abortive treatment is closer to the standard of care for severe headaches. The nonpharmacologic approaches delineated in this article are the mainstay of treatment.
Prophylactic pharmacotherapy for migraine would be more justifiable if it also treated other conditions in which the risks/benefits for both the mother and fetus were clearer, such as maternal hypertension (in which labetalol can be effective for both conditions), or severe depression. Other nontraditional therapies that have shown efficacy for nonpregnant patients such as magnesium supplementation may be worthy of study since the risk of fetal harm in all trimesters appears remote.
In all cases, carefully documenting patient involvement in risk/benefit discussions.
Evidence summary
Eighteen percent of all women report migraines.1 Among pregnant migraineurs, 2.5% to 8% reported worsening symptoms.1,2 Guidelines recommend considering prophylaxis for nonpregnant patients if they experience at least 3 or 4 prolonged severe attacks per month.3
Nonpharmacological treatment. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed alleviation of symptoms for 15 of 19 women. The second, a small unblinded RCT, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. Over 72% of the treatment arm improved compared with nearly 29% of the control group.4 The 30 women (19 from the original cohort and the 11 from the intervention arm of the RCT) were then followed as a cohort for the duration of pregnancy and 1 year postpartum. More than 67% of the patients continued to report a decrease in the frequency and severity of headache.5 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Pharmacologic agents. Randomized controlled trials have demonstrated that multiple medications have prophylactic benefit in the treatment of nonpregnant patients with migraine. In particular, propanolol,6 divalproex sodium/sodium valproate, and topiramate7 have been effective. A single case report on the use of labetalol by a pregnant woman at 28 weeks’ gestational age showed that it was effective in reducing the frequency and severity of her headaches after 1 week of use. This improvement persisted until delivery at 38 weeks.8
Safety in pregnancy. The Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.9 There are no data about the effectiveness of medications for migraine prophylaxis in pregnancy so one cannot select a specific medication with certainty. However, it may be reasonable to select medications based on both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary.
The TABLE shows commonly used drugs for prophylaxis of migraine and their pregnancy risk category classification. It should be noted that even if risk has been demonstrated in a medication, not all risks are equal. For example, propanolol is class D because of increased risk for intrauterine growth restriction in the third trimester, while sodium valproate is class D because of known teratogenicity.9
TABLE
Pregnancy risk category of some prophylactic drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Labetalol (Normodyne, Trandate) | C/D* |
| Propanolol (Inderal, Inderide) | C/D* |
| Verapamil (Calan, Isoptin, Verelan) | C |
| Nifedipine (Procardia) | C |
| Amitriptyline (Limbitrol) | C |
| Nortriptyline (Aventyl, Pamelor) | D |
| Fluoxetine (Prozac) | C |
| Gabapentin (Neurontin) | C |
| Divalproex sodium (Depakote) | D |
| Topiramate (Topama x) | C |
| A=controlled human studies show no risk, B=No evidence of risk in humans, but no controlled studies, C=Risk to humans has not been rule d out, D=Positive evidence of risk to humans from human or animal studies, X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.9 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend avoidance of prophylactic medications in pregnancy, if possible. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy. If drug treatment is necessary, they recommend selecting an agent with the lowest risk of adverse effects to the fetus.3 Most review articles state that, if medication is necessary, it should be tailored towards other comorbidities, if possible; if there are no coexisting conditions, then calcium channel blockers or beta blockers would be the treatment of choice, based on safety data.1,10
No randomized controlled trials (RCT) have addressed pharmacologic prophylaxis of migraine for pregnant women. Two studies suggest that nonpharmacologic therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) not only relieved acute pain, but also decreased the frequency of headaches (strength of recommendation [SOR]: B, poor-quality cohort and RCTs).
Practice guidelines and most review articles recommend avoiding prophylactic medications if possible. If a medication must be used, base the selection on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies (SOR: C, expert opinion).
Nonpharmacologic approaches, limited analgesics remain the mainstay of prevention for pregnant women
Robert Sheeler, MD
Mayo Clinic, Rochester, Minn
Standards of care favor minimizing any use of drugs with known or even theoretical risk to the fetus. That includes virtually all the classes of drugs prescribed for migraine prevention. Limited use of analgesics such as acetaminophen and opioids for migraine-abortive treatment is closer to the standard of care for severe headaches. The nonpharmacologic approaches delineated in this article are the mainstay of treatment.
Prophylactic pharmacotherapy for migraine would be more justifiable if it also treated other conditions in which the risks/benefits for both the mother and fetus were clearer, such as maternal hypertension (in which labetalol can be effective for both conditions), or severe depression. Other nontraditional therapies that have shown efficacy for nonpregnant patients such as magnesium supplementation may be worthy of study since the risk of fetal harm in all trimesters appears remote.
In all cases, carefully documenting patient involvement in risk/benefit discussions.
Evidence summary
Eighteen percent of all women report migraines.1 Among pregnant migraineurs, 2.5% to 8% reported worsening symptoms.1,2 Guidelines recommend considering prophylaxis for nonpregnant patients if they experience at least 3 or 4 prolonged severe attacks per month.3
Nonpharmacological treatment. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed alleviation of symptoms for 15 of 19 women. The second, a small unblinded RCT, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. Over 72% of the treatment arm improved compared with nearly 29% of the control group.4 The 30 women (19 from the original cohort and the 11 from the intervention arm of the RCT) were then followed as a cohort for the duration of pregnancy and 1 year postpartum. More than 67% of the patients continued to report a decrease in the frequency and severity of headache.5 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Pharmacologic agents. Randomized controlled trials have demonstrated that multiple medications have prophylactic benefit in the treatment of nonpregnant patients with migraine. In particular, propanolol,6 divalproex sodium/sodium valproate, and topiramate7 have been effective. A single case report on the use of labetalol by a pregnant woman at 28 weeks’ gestational age showed that it was effective in reducing the frequency and severity of her headaches after 1 week of use. This improvement persisted until delivery at 38 weeks.8
Safety in pregnancy. The Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.9 There are no data about the effectiveness of medications for migraine prophylaxis in pregnancy so one cannot select a specific medication with certainty. However, it may be reasonable to select medications based on both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary.
The TABLE shows commonly used drugs for prophylaxis of migraine and their pregnancy risk category classification. It should be noted that even if risk has been demonstrated in a medication, not all risks are equal. For example, propanolol is class D because of increased risk for intrauterine growth restriction in the third trimester, while sodium valproate is class D because of known teratogenicity.9
TABLE
Pregnancy risk category of some prophylactic drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Labetalol (Normodyne, Trandate) | C/D* |
| Propanolol (Inderal, Inderide) | C/D* |
| Verapamil (Calan, Isoptin, Verelan) | C |
| Nifedipine (Procardia) | C |
| Amitriptyline (Limbitrol) | C |
| Nortriptyline (Aventyl, Pamelor) | D |
| Fluoxetine (Prozac) | C |
| Gabapentin (Neurontin) | C |
| Divalproex sodium (Depakote) | D |
| Topiramate (Topama x) | C |
| A=controlled human studies show no risk, B=No evidence of risk in humans, but no controlled studies, C=Risk to humans has not been rule d out, D=Positive evidence of risk to humans from human or animal studies, X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.9 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend avoidance of prophylactic medications in pregnancy, if possible. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy. If drug treatment is necessary, they recommend selecting an agent with the lowest risk of adverse effects to the fetus.3 Most review articles state that, if medication is necessary, it should be tailored towards other comorbidities, if possible; if there are no coexisting conditions, then calcium channel blockers or beta blockers would be the treatment of choice, based on safety data.1,10
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 2004;22:727-756.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence base d review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Scharff L, Marcus DA, Turk DC. Maintenance of effects in the nonmedical treatment of headaches during pregnancy. Headache 1996;36:285-290
6. Linde K, Rossnagel K. Propanolol for migraine prophylaxis. Cochrane Database Syst Rev 2004;(2):CD003225-
7. Chronicle E, Mulleners W. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev 2004;(3):CD003226-
8. Dey R, Khan S, Akhouri V, Wootton J, Bajwa Z. Labetalol for prophylactic treatment of intractable migraine during pregnancy. Headache 2002;42:642-645.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins, 2002.
10. Martin SR, Foley MR. Approach to the pregnant patient with headache. Clin Obstet Gynecol 2005;48:2-11.
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 2004;22:727-756.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence base d review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Scharff L, Marcus DA, Turk DC. Maintenance of effects in the nonmedical treatment of headaches during pregnancy. Headache 1996;36:285-290
6. Linde K, Rossnagel K. Propanolol for migraine prophylaxis. Cochrane Database Syst Rev 2004;(2):CD003225-
7. Chronicle E, Mulleners W. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev 2004;(3):CD003226-
8. Dey R, Khan S, Akhouri V, Wootton J, Bajwa Z. Labetalol for prophylactic treatment of intractable migraine during pregnancy. Headache 2002;42:642-645.
9. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins, 2002.
10. Martin SR, Foley MR. Approach to the pregnant patient with headache. Clin Obstet Gynecol 2005;48:2-11.
Evidence-based answers from the Family Physicians Inquiries Network
What are the best therapies for acute migraine in pregnancy?
No randomized controlled trials of pharmacologic therapy for acute migraine in pregnant women are available. Three treatment studies suggest that nonpharmacological therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) were effective for pain relief (strength of recommendation [SOR]: C, poor-quality cohort and case-control studies). Practice guidelines and most review articles recommend acetaminophen as the first-line therapy (SOR: C, expert opinion). Treatment modalities, including medications, should be chosen based on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies.
It is helpful to test nonpharmacologic treatments during the prepregnancy period
Tricia C. Elliott, MD
Department of Family Medicine, Baylor College of Medicine, Houston, Tex
For young women diagnosed with migraine, begin discussing with them during the family planning period treatment options for acute migraine in pregnancy. Trials of approved medications and nonpharmacologic treatments can be given at this time to evaluate their efficacy and to give the patient time to feel comfortable with them. It is especially important to test nonpharmacologic treatments during the prepregnancy period.
In my own experience as a physician, and as a young woman with a long-standing history of migraine, biofeedback and relaxation techniques work better when the patient is first exposed during pain-free or subacute pain periods. For moderate to severe migraineurs, it is difficult to institute these techniques during a full-blown attack. For such patients, experience with safer treatment modalities before pregnancy would allow greater success for treatment of acute migraine during pregnancy.
Evidence summary
Eighteen percent of all women report migraines.1 As estrogen levels increase early in pregnancy, many women report an increase in headache or new-onset headache. As estrogen levels stabilize in the second and third trimester, 60% to 70% of women with migraine report reduction in symptoms.1,2
Nonpharmacologic treatment. A small case series of electromyograph (EMG) biofeedback and relaxation techniques on 5 pregnant women showed that 4 became headache-free.3 It is impossible to say whether it was the intervention, natural disease progression, or the attention received from the therapist that produced this result. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed decrease in symptoms for 15 of 19 women. The second, a small randomized controlled trial, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. More than 72% of the treatment arm improved, compared with nearly 29% of the attention control group.4 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Sumatriptan and other agents. Six studies have evaluated sumatriptan use in pregnancy.5 All were designed to evaluate teratogenicity and harm. None evaluated treatment efficacy in pregnancy. One prospective controlled cohort study showed an increase in miscarriage rates that did not reach statistical significance.6 No trials showed an increased risk in birth defects compared with the general population.
A single case report on the use of intravenous magnesium sulfate and prochlorperazine reported that the combination was effective for aborting a prolonged (6-day) migraine with aura for a pregnant woman.7
Safety in pregnancy. The US Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.8 Though no data exist on the effectiveness of other medications for migraine in pregnancy, it is reasonable to select drugs for both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary. The TABLE shows commonly used drugs for acute migraine and their pregnancy risk category classification.
TABLE
Pregnancy risk category of abortive drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Acetaminophen | B |
| Ibuprofen | B/D* |
| Naproxen | B/D* |
| Acetaminophen/oxycodone | B |
| Acetaminophen/codeine | C |
| Meperidine | B |
| Prochlorperazine | C |
| Sumatriptan | C |
| Butalbital/aspirin/caffeine | C/D* |
| Ergotamine/caffeine | X |
| A=Controlled human studies show no risk; B=No evidence of risk in humans, but no controlled studies; C=Risk to humans has not been ruled out; D=Positive evidence of risk to humans from human or animal studies; X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.7 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend acetaminophen as first-line therapy based on its established safety in surveillance studies, although it is of questionable efficacy for nonpregnant patients. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy.9 Most review articles also recommend acetaminophen alone or in combination with codeine as the treatment of first choice.1,10
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 1997;15:209-231.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Hickling EJ, Silverman DJ, Loos W. A non-pharmacological treatment of vascular headache during pregnancy. Headache 1990;30:407-410.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Hilaire ML, Cross LB, Eichner SF. Treatment of migraine headaches with sumatriptan in pregnancy. Ann Pharmacother 2004;38:1726-1730.
6. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology 1998;51:581-583.
7. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins; 2002.
8. Rozen TD. Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. Headache 2003;43:901-903.
9. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
10. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med 2004;115:39-44,47-50.
No randomized controlled trials of pharmacologic therapy for acute migraine in pregnant women are available. Three treatment studies suggest that nonpharmacological therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) were effective for pain relief (strength of recommendation [SOR]: C, poor-quality cohort and case-control studies). Practice guidelines and most review articles recommend acetaminophen as the first-line therapy (SOR: C, expert opinion). Treatment modalities, including medications, should be chosen based on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies.
It is helpful to test nonpharmacologic treatments during the prepregnancy period
Tricia C. Elliott, MD
Department of Family Medicine, Baylor College of Medicine, Houston, Tex
For young women diagnosed with migraine, begin discussing with them during the family planning period treatment options for acute migraine in pregnancy. Trials of approved medications and nonpharmacologic treatments can be given at this time to evaluate their efficacy and to give the patient time to feel comfortable with them. It is especially important to test nonpharmacologic treatments during the prepregnancy period.
In my own experience as a physician, and as a young woman with a long-standing history of migraine, biofeedback and relaxation techniques work better when the patient is first exposed during pain-free or subacute pain periods. For moderate to severe migraineurs, it is difficult to institute these techniques during a full-blown attack. For such patients, experience with safer treatment modalities before pregnancy would allow greater success for treatment of acute migraine during pregnancy.
Evidence summary
Eighteen percent of all women report migraines.1 As estrogen levels increase early in pregnancy, many women report an increase in headache or new-onset headache. As estrogen levels stabilize in the second and third trimester, 60% to 70% of women with migraine report reduction in symptoms.1,2
Nonpharmacologic treatment. A small case series of electromyograph (EMG) biofeedback and relaxation techniques on 5 pregnant women showed that 4 became headache-free.3 It is impossible to say whether it was the intervention, natural disease progression, or the attention received from the therapist that produced this result. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed decrease in symptoms for 15 of 19 women. The second, a small randomized controlled trial, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. More than 72% of the treatment arm improved, compared with nearly 29% of the attention control group.4 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Sumatriptan and other agents. Six studies have evaluated sumatriptan use in pregnancy.5 All were designed to evaluate teratogenicity and harm. None evaluated treatment efficacy in pregnancy. One prospective controlled cohort study showed an increase in miscarriage rates that did not reach statistical significance.6 No trials showed an increased risk in birth defects compared with the general population.
A single case report on the use of intravenous magnesium sulfate and prochlorperazine reported that the combination was effective for aborting a prolonged (6-day) migraine with aura for a pregnant woman.7
Safety in pregnancy. The US Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.8 Though no data exist on the effectiveness of other medications for migraine in pregnancy, it is reasonable to select drugs for both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary. The TABLE shows commonly used drugs for acute migraine and their pregnancy risk category classification.
TABLE
Pregnancy risk category of abortive drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Acetaminophen | B |
| Ibuprofen | B/D* |
| Naproxen | B/D* |
| Acetaminophen/oxycodone | B |
| Acetaminophen/codeine | C |
| Meperidine | B |
| Prochlorperazine | C |
| Sumatriptan | C |
| Butalbital/aspirin/caffeine | C/D* |
| Ergotamine/caffeine | X |
| A=Controlled human studies show no risk; B=No evidence of risk in humans, but no controlled studies; C=Risk to humans has not been ruled out; D=Positive evidence of risk to humans from human or animal studies; X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.7 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend acetaminophen as first-line therapy based on its established safety in surveillance studies, although it is of questionable efficacy for nonpregnant patients. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy.9 Most review articles also recommend acetaminophen alone or in combination with codeine as the treatment of first choice.1,10
No randomized controlled trials of pharmacologic therapy for acute migraine in pregnant women are available. Three treatment studies suggest that nonpharmacological therapies (combinations of skin warming, relaxation, biofeedback, and physical therapy) were effective for pain relief (strength of recommendation [SOR]: C, poor-quality cohort and case-control studies). Practice guidelines and most review articles recommend acetaminophen as the first-line therapy (SOR: C, expert opinion). Treatment modalities, including medications, should be chosen based on both effectiveness for nonpregnant patients and established pregnancy safety from surveillance studies.
It is helpful to test nonpharmacologic treatments during the prepregnancy period
Tricia C. Elliott, MD
Department of Family Medicine, Baylor College of Medicine, Houston, Tex
For young women diagnosed with migraine, begin discussing with them during the family planning period treatment options for acute migraine in pregnancy. Trials of approved medications and nonpharmacologic treatments can be given at this time to evaluate their efficacy and to give the patient time to feel comfortable with them. It is especially important to test nonpharmacologic treatments during the prepregnancy period.
In my own experience as a physician, and as a young woman with a long-standing history of migraine, biofeedback and relaxation techniques work better when the patient is first exposed during pain-free or subacute pain periods. For moderate to severe migraineurs, it is difficult to institute these techniques during a full-blown attack. For such patients, experience with safer treatment modalities before pregnancy would allow greater success for treatment of acute migraine during pregnancy.
Evidence summary
Eighteen percent of all women report migraines.1 As estrogen levels increase early in pregnancy, many women report an increase in headache or new-onset headache. As estrogen levels stabilize in the second and third trimester, 60% to 70% of women with migraine report reduction in symptoms.1,2
Nonpharmacologic treatment. A small case series of electromyograph (EMG) biofeedback and relaxation techniques on 5 pregnant women showed that 4 became headache-free.3 It is impossible to say whether it was the intervention, natural disease progression, or the attention received from the therapist that produced this result. Two studies were published together evaluating thermal biofeedback, relaxation training, and physical therapy exercises. The first, a cohort study, showed decrease in symptoms for 15 of 19 women. The second, a small randomized controlled trial, compared 11 women using the combination treatment with 14 control women who received attention from the therapist but no other intervention. More than 72% of the treatment arm improved, compared with nearly 29% of the attention control group.4 Interpretation of these studies is limited by small sample size and testing in settings with specialized resources that are not found in every community.
Sumatriptan and other agents. Six studies have evaluated sumatriptan use in pregnancy.5 All were designed to evaluate teratogenicity and harm. None evaluated treatment efficacy in pregnancy. One prospective controlled cohort study showed an increase in miscarriage rates that did not reach statistical significance.6 No trials showed an increased risk in birth defects compared with the general population.
A single case report on the use of intravenous magnesium sulfate and prochlorperazine reported that the combination was effective for aborting a prolonged (6-day) migraine with aura for a pregnant woman.7
Safety in pregnancy. The US Food and Drug Administration (FDA) assigns fetal risk categories to all drugs based on controlled studies in humans, animal reproduction studies, and surveillance studies.8 Though no data exist on the effectiveness of other medications for migraine in pregnancy, it is reasonable to select drugs for both effectiveness for nonpregnant patients and established safety as determined by the FDA’s fetal risk summary. The TABLE shows commonly used drugs for acute migraine and their pregnancy risk category classification.
TABLE
Pregnancy risk category of abortive drugs for migraine
| MEDICATION | PREGNANCY RISK CATEGORY |
|---|---|
| Acetaminophen | B |
| Ibuprofen | B/D* |
| Naproxen | B/D* |
| Acetaminophen/oxycodone | B |
| Acetaminophen/codeine | C |
| Meperidine | B |
| Prochlorperazine | C |
| Sumatriptan | C |
| Butalbital/aspirin/caffeine | C/D* |
| Ergotamine/caffeine | X |
| A=Controlled human studies show no risk; B=No evidence of risk in humans, but no controlled studies; C=Risk to humans has not been ruled out; D=Positive evidence of risk to humans from human or animal studies; X=Contraindicated in pregnancy. | |
| *Category changes to D if used in 3rd trimester. | |
| Source: Briggs et al 2002.7 | |
Recommendations from others
Practice guidelines published by the American Academy of Neurology recommend acetaminophen as first-line therapy based on its established safety in surveillance studies, although it is of questionable efficacy for nonpregnant patients. They also recommend nonpharmacologic treatment as an acceptable option in pregnancy.9 Most review articles also recommend acetaminophen alone or in combination with codeine as the treatment of first choice.1,10
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 1997;15:209-231.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Hickling EJ, Silverman DJ, Loos W. A non-pharmacological treatment of vascular headache during pregnancy. Headache 1990;30:407-410.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Hilaire ML, Cross LB, Eichner SF. Treatment of migraine headaches with sumatriptan in pregnancy. Ann Pharmacother 2004;38:1726-1730.
6. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology 1998;51:581-583.
7. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins; 2002.
8. Rozen TD. Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. Headache 2003;43:901-903.
9. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
10. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med 2004;115:39-44,47-50.
1. Silberstein SD. Migraine and pregnancy. Neurol Clin 1997;15:209-231.
2. Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia 1997;17:765-769.
3. Hickling EJ, Silverman DJ, Loos W. A non-pharmacological treatment of vascular headache during pregnancy. Headache 1990;30:407-410.
4. Marcus DA, Scharff L, Turk DC. Nonpharmacological management of migraines in pregnancy. Psychosom Med 1995;57:527-535.
5. Hilaire ML, Cross LB, Eichner SF. Treatment of migraine headaches with sumatriptan in pregnancy. Ann Pharmacother 2004;38:1726-1730.
6. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology 1998;51:581-583.
7. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 6th ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins; 2002.
8. Rozen TD. Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. Headache 2003;43:901-903.
9. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
10. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med 2004;115:39-44,47-50.
Evidence-based answers from the Family Physicians Inquiries Network
Evaluation and treatment of the patient with allergic rhinitis
- Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”).
- Skin prick testing can detect IgE antibodies in patients with reliable histories of exposure to allergens.
- Intranasal corticosteroids are superior to other medications in achieving desired clinical outcomes, including quality of life.
- For some cases of allergic rhinitis, subcutaneous immunotherapy can achieve clinical remission for up to 3 years after cessation of therapy.
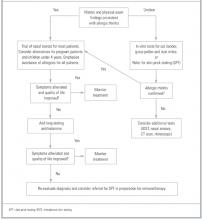
While allergic rhinitis is merely a nuisance to most people afflicted by it, the condition can lead to complications if it is severe or exists undetected for too long. In this article, I review the most reliable means of diagnosing allergic rhinitis, and outline a recommended approach to treatment.
Prevalence and pathophysiology
An estimated 20 to 40 million Americans are affected by allergic rhinitis. The actual prevalence of the condition is difficult to discern as many sufferers self-medicate without seeking medical care. One survey stated that up to 92% of patients had self-medicated prior to seeking medical care.1 Even when accounting for self-treatment, allergic rhinitis is the most commonly encountered form of chronic rhinitis, representing about 3% of all primary care office visits.2,3 Direct and indirect clinical costs run between $1.2 and $5.3 billion per year.4-6 Although the disease can develop in persons of any age, in 80% of cases symptoms will develop before the patient is 20 years old.5 Symptoms often wane as a patient grows older, and it is uncommon for persons older than 65 to experience new onset of allergic rhinitis.3,7
Allergic rhinitis stems from a type I hypersensitivity reaction.4 During an initial sensitization phase, the immune system identifies an allergen as foreign and generates specific antibodies to act against that allergen. Atopic patients exhibit an exaggerated response, generating high levels of Type 2 T-helper (Th2) cells and, subsequently, IgE antibodies.8 On reexposure to the allergen, specific IgE antibodies bound to mast cells form cross-links resulting in mast cell degranulation and the release of histamine and other chemical mediators. The patient then immediately develops such allergy symptoms as itching, sneezing, and rhinorrhea. A cellular inflammatory response, chiefly involving eosinophils, monocytes, and basophils, characterizes the secondary phase of the allergic reaction. Nasal congestion tends to dominate this later response phase.
Seasonal allergic rhinitis is usually triggered by pollens or molds. Perennial allergic rhinitis, triggered by dust mites, molds, cockroach or animal allergens, is defined as occurring 9 months out of the year.9
Clinical evaluation
The diagnosis of allergic rhinitis is usually made on the basis of the patient’s history and the results of your physical examination. In addition to classic symptoms of nasal congestion, itchy nose, sneezing, rhinorrhea, or itchy, watery eyes, patients may also complain of chronic cough, dry scratchy throat, otalgia, or recurrent sinusitis.4 Other important historical considerations include a family history of allergic rhinitis, a history of other atopic disease, previous treatment experiences, and suspected triggers.5
Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”). Chronic nasal congestion may also precipitate darkening of the skin under the eyes or “allergic shiners.”2,6 Concurrent conjunctivitis is common. Polyps, seen on direct nasal examination, may occur both in allergic and non-allergic patients.
No studies have evaluated the accuracy of the history or physical examination in confirming the diagnosis of allergic rhinitis. The differential diagnosis is extensive and includes infectious rhinitis, non-allergic rhinitis with eosinophilia syndrome (NARES), occupational rhinitis, mechanical obstruction, vasomotor rhinitis, drug-induced rhinitis, and nasal polyps.5
Diagnostic tests
Published guidelines from the American Academy of Asthma, Allergy and Immunology, as well as other expert panels, recommend confirmatory testing when allergic rhinitis is clinically suspected.2,5,10 There is no evidence to support the superiority of this recommendation over an empiric trial of medication, and most primary care physicians choose to treat empirically based upon the history and physical examination.
Although further testing should be done when the diagnosis is unclear, be aware that there is uncertainty associated with allergy testing. Because an individual may become sensitized to an allergen without exhibiting symptoms of allergic rhinitis, there is no clearly defined reference standard for the confirmation of allergic rhinitis.11 Likewise, a history of sensitivity is not always followed by expected IgE test results. Challenge methods developed for studies of airborne allergens are used as reference standards in the evaluation of clinical tests.12
Diagnostic tests include skin prick testing, intradermal testing, and in vitro blood tests. Nasal challenge testing, nasal smears, sinus transillumination, and nasopharyngoscopy are nonspecific tests. They are not recommended for routine evaluation but may be useful in selected cases when allergen-specific tests have failed to clarify the cause of the rhinitis. An expert panel has stated that no studies address the cost-effectiveness of any of these methods.2
Skin prick testing (SPT) is considered the most convenient and least expensive screening test. SPT can detect IgE antibodies in patients with reliable exposure histories.13 Sensitivity and specificity are difficult to determine, for a number of reasons. First, as previously mentioned, there is no clearly defined reference standard.11 Second, only 5 allergen extracts have been standardized for defined quantities known to induce biologic activity. Standardized extracts in the United States include ragweed pollen, cat dander, house dust mites, Hymenoptera venoms, and some grasses. All other extracts are local or regional preparations, and skin tests with nonstandard extracts are not necessarily reproducible.11 Third, even with a single individual, there can be wide variation in skin reaction to the same reagent, depending on the device used.14 As a result, correlation between SPT and inhalation challenges vary from 60% to 90%.13
Intradermal skin tests (IDST) are usually done when SPT yields a negative result despite a history compatible with allergic rhinitis.13 The primary advantage of IDST is sensitivity afforded by a fixed concentration of allergen. Because of this sensitivity, not all reactions are clinically relevant.13 In fact, IDST is often used as a reference standard in studies of the accuracy of SPT and in vitro tests.
Several in vitro assays of specific IgE antibodies are available. They are all modeled after the original radioallergosorbent tests (RAST); the term “RAST” is often used interchangeably with any type of in vitro blood test.13 IgE antibody tests have a high false-positive rate, meaning the test is positive in patients without allergy symptoms. RAST tests are less sensitive than SPT, with a mean sensitivity of 75% and a range of approximately 50% to 95%.13
The 3 primary diagnostic tests for allergic rhinitis are usually compared with each other and not to a recognized standard. Table 1 summarizes data from a study that compared all 3 tests with subjects who were placed in a small room with 2 cats and their bed.12 While this is one of very few studies that contrasts all 3 tests to a reasonable reference standard, the findings cannot necessarily be extrapolated to other airborne allergens.
In the hope of limiting referrals to allergists for testing, and reducing the uncertainty in making a diagnosis, one study looked at the RAST response to 19 allergens. The authors found that of all the patients who responded to any allergen, 95% exhibited responses specifically to grass pollen, dust mites, or cat dander. They went on to conclude that 96.3% of patients with allergic disease could be correctly identified with a combination of a standardized history (available in the study text), a total serum IgE of greater than 40 U/mL, and in vitro tests for cat dander, dust mites, and grass pollen.15
TABLE 1
Accuracy of diagnostic tests for diagnosis of cat allergy13
| Test | Sensitivity | Specificity | PV+ | PV- | LR+ | LR- |
|---|---|---|---|---|---|---|
| Skin prick test | 79.2 | 90.6 | 92.6 | 74.3 | 8.4 | 0.2 |
| Intradermal test | 60.0 | 31.0 | 23.1 | 69.2 | 0.9 | 1.3 |
| RAST | 69.2 | 100 | 100 | 72.7 | 69.2 | 0.3 |
| Note: Results are based upon any upper or lower symptoms when exposed to cat challenge. Intradermal test done if negative skin prick test. LR+ = positive likelihood ratio, LR- = negative likelihood ratio, PV+ = positive predictive value, PV- = negative predictive value. | ||||||
Treatment
Untreated allergic rhinitis can have a significant impact on quality of life. Patients are bothered by nose blowing, disrupted sleep, fatigue, and decreased concentration.1 In one 1996 survey, 32 % of patients said that allergy attacks embarrassed them or interfered with their quality of life.16 As a result, most patient-oriented studies on treatment evaluate the impact on health-related quality of life.17
The initial form of treatment is usually avoidance of the allergen, although this can be difficult. For animal allergens, washing pets and using high-efficiency particulate air (HEPA) filters have been shown to temporarily reduce the volume of airborne allergens but not to improve patient-oriented outcomes.18,19 Removing the pet from the home is the only sure remedy.18 More studies are needed to evaluate the benefit of multiple home treatments to reduce exposure to cockroach and fungal allergens.18 A systematic review of several studies showed that maternal antigen avoidance during lactation reduced the incidence of atopic dermatitis in at-risk infants.20 A meta-analysis of measures to avoid house-dust mites showed no clear benefit for patients with asthma10 It is unclear if these findings can be extrapolated to other atopic conditions such as allergic rhinitis.
Intranasal corticosteroids. Intranasal corticosteroids are the most effective medication in the treatment of allergic rhinitis. Available preparations in the US include beclomethasone diproprionate, budesonide, funisolide, fluticasone propionate, mometa-sone furoate, and triamcinalone acetonide. A meta-analysis identified 16 randomized controlled trials (RCTs) that compared antihistamines with intranasal corticosteroids in a total of 2767 patients. Intranasal corticosteroids provided significantly greater relief from nasal discharge, sneezing, pruritis, and postnasal drip. There was no statistically significant difference between the 2 in reduction of eye symptoms.21
Although this review did not address quality of life, other studies have shown that both triamcinolone acetonide and fluticasone propionate are superior to loratadine in improving quality of life.22,23 Few studies provide any guidance in choosing one intranasal steroid over another. Generally, they are of equal efficacy in patient-oriented outcomes.24,25 Although intranasal corticosteroids are considered daily or “maintenance” medications, a single small RCT of 26 patients showed that fluticasone propionate improved quality of life and reduced symptoms compared with placebo when used on an as-needed basis over a 4-week period.26 More studies are needed to confirm this preliminary finding, though.
Antihistamines. Although not as effective as intranasal steroids, antihistamines do reduce symptoms of rhinorrhea, sneezing, and itching.27 First-gen-eration antihistamines (diphenhydramine, chlorpheniramine, etc.) are lipophilic and cross the blood-brain barrier, resulting in varying degrees of anticholinergic side effects. Placebo-controlled studies have confirmed that these agents cause psychomotor retardation, sleepiness, and decreased work production.5,28 Specifically they seem to affect attention, memory, and vigilance. These symptoms may persist even after an overnight period of sleep.28,29 Second-generation antihistamines (fexofenadine, loratadine, etc.) do not penetrate the brain as well and are less likely to cause central nervous system effects.
However, a recent RCT involving 63 elementary school students challenges findings from previous studies. Children who received diphenhydramine, 25 mg twice daily, performed no differently on computerized reaction-time tests or multiple-choice learning tests than did children who received placebo or loratadine, 10 mg daily.30 Another RCT involving 845 patients from ages 12 to 65 years evaluated quality of life as well as work and school performance of patients who received fexofenadine or placebo. While quality-of-life scores and work performance improved significantly with fexofenadine, there was no significant difference between the groups in school performance.31 Direct comparisons of antihistamines are rare and the results are conflicting. There are no data to show that one of the first-generation antihistamines is superior to the others. Similarly, second-generation drugs are no more effective than the older medications; they only have fewer side effects. Among the second-generation antihistamines, fexofenadine and cetirizine appear to be more effective then loratadine.29
Decongestants. Systemic and topical decongestants relieve the congestion that accompanies the secondary phase of an allergic reaction.4 They have limited effects on other allergic symptoms and, as a result, are often used in combination with antihistamines.32 When used for more than 10 days, topical decongestants (oxymetazoline, xylometazoline) are associated with rebound congestion (rhinitis medicamentosa).33
Leukotriene receptor antagonists. Although not approved by the FDA for treatment of allergic rhinitis, the leukotriene receptor antagonist montelukast was shown in a randomized double-blinded trial to be as effective as loratadine in relieving symptoms. There was minimal additional benefit in using the medications concomitantly.34
Cromolyn sodium. Cromolyn sodium has been shown to prevent the onset of allergic rhinitis symptoms in multiple placebo-controlled trials.35 It is extremely safe but requires regular use and is not as effective as other medications for acute symptoms. Direct comparison studies have shown that cromolyn is not as effective as intranasal corticosteroids.35,36
Immunotherapy. Subcutaneous immunotherapy (SIT) is recommended by all guidelines for patients who fail to respond to pharmacotherapy and allergen avoidance.2,5,10,27 It is recommended in particular for allergic rhinitis secondary to ragweed, grasses, molds, and dust mites. Immunotherapy induces the creation of protective IgG and inhibits the inflammatory response to allergens.27 SIT requires specific allergen confirmation with either a skin test or in vitro assay. Preparation of SIT doses should be done by a practitioner well trained in mixing and diluting extracts.5,37 Forty-three placebo-controlled, double-blind studies have evaluated the efficacy of SIT for 12 different allergens since 1980.10 Thirty-two trials showed clinical efficacy, which can be long lasting. A study of patients treated for 3 to 4 years with immunotherapy for grass pollen allergy showed continued clinical remission for at least 3 years after treatment was stopped.38
Herbal therapies. Alternative approaches to the treatment of allergic rhinitis warrant further investigation. Herbal medications, such as licorice, gingko, and ginseng, are currently used to treat allergic rhinitis, although there are no large studies to confirm their effectiveness.39
Probiotics. Epidemiologic studies suggest that the increase in atopic disease may be related to a clean environment and widespread use of antibiotics in Western countries. The environment may deprive fetal and infant immune systems of bacterial antigens that stimulate type 1 T-helper (Th1) cells.8 In light of this theory, Finnish researchers randomly assigned 159 pregnant women with a family history of atopy to receive capsules of Lactobacillus GG (a potentially beneficial bacteria or “probiotic”) or placebo, beginning 2 to 4 weeks prior to delivery and continuing 6 months postpartum. Infants were followed for 2 years. Frequency of atopic dermatitis was reduced by 50% among those infants whose mothers received Lactobacillus.40 Further study of this association in allergic rhinitis would be beneficial.
Treatment recommendations.Table 2 summarizes treatment-related evidence in the management of allergic rhinitis, and the Figure illustrates a proposed treatment algorithm. Another algorithm by the European Academy of Allergy and Clinical Immunology recommends initial therapy with oral or nasal antihistamines for mild disease, nasal corticosteroids for moderate disease, and both for severe disease.10 This was a consensus opinion. Of the 2 most commonly used medications for allergy, nasal steroids are favored over antihistamines for overall safety, tolerability, effectiveness, and simplicity in all cases. In one study of 61 adults, patients were randomized to receive either a nasal steroid or an antihistamine as initial therapy, with the other agent reserved as “back-up.” After 6 weeks, 86% of patients started on an antihistamine had added their steroid back-up, while 51% of the group started on a steroid remained on that agent alone.41 Starting all patients on both an antihistamine and a nasal steroid is inappropriate.
TABLE 2
Evidence to support treatment recommendations
| Strength of recommendation | Treatment | Comment |
|---|---|---|
| A | Immunotherapy | Can have long lasting clinical benefit. |
| A | Intranasal Consistently | superior to antihistamines corticosteroids in head to head trials. Not clear if all steroids are equally effective. |
| A | Antihistamines | Effective, but inferior to intranasal steroids in most clinical outcomes. |
| A | Cromolyn sodium | Intranasal steroids superior in all clinical outcomes. |
| B | Decongestants | Less effective than antihistamines in direct comparisons, many trials involve combination products. |
| D | Probiotics | Larger trials needed, limited evidence. |
| D | Herbal medications (licorice, gingko, ginseng) | Limited evidence. |
FIGUREA guide to evaluation and treatment of allergic rhinitis
Prognosis
The long-term prognosis for allergic rhinitis is excellent. For most patients, the illness is primarily a nuisance with no significant morbidity. However, for patients whose rhinitis is moderate to severe and poorly controlled, there can be significant complications. These complications include asthma, sinusitis, otitis media, nasal polyposis, respiratory infections, and orthodontic malocclusions.42 In one study of 605 children with allergic rhinitis, 21% had chronic otitis media with effusion (OME). Conversely, in another study of 259 children with OME, 50% had allergic rhinitis.43 Even among patients without asthma, 20% to 30% will have bronchial hyper-responsiveness. Additionally, poorly controlled allergic rhinitis can contribute to sleep loss, daytime fatigue, and learning impairment.44
Potential complications related to long-term treatment in children remain controversial. In a 1998 study of intranasal beclomethasone, children receiving the study medication grew an average of only 5 centimeters (cm) in 1 year, compared with an average of 5.9 cm in the placebo group.45 However, a similar study done 2 years later with intranasal mometa-sone showed no evidence of growth suppression.45 Further studies are needed before the true impact of intranasal steroids on children can be determined.
· Acknowledgments ·
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. The author wishes to thank Kathleen Conner, JD, for her assistance in the preparation of the manuscript.
1. Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S805-28.
2. Fornadley JA, et al. Allergic rhinitis: Clinical practice guideline. Otolaryngol Head Neck Surg 1996;115(1):115-122.
3. Wagner W, Kavuru M. Diagnosis and Management of Rhinitis. 1st Ed. Professional Communications, Inc. 1996. Available at www.medscape.com. Accessed Sept. 6, 2001.
4. Hadley JA. Evaluation and management of allergic rhinitis. Med Clin North Am 1999;83(1):13-25.
5. Dykewicz MS, Fineman S, et al. Diagnosis and management of rhinitis: complete guidelines of the joint task force on practice parameters in allergy, asthma and immunology. Ann Allergy Asthma Immunol 1998;81:478-518.
6. Weiss KB. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001;107:3-8.
7. Gentile DA, Friday GA, Skoner DP. Management of allergic rhinitis: antihistamines and decongestants. Immunol Allergy Clin North Am 2000;20(2):355-368.
8. Kay AB. Allergy and allergic diseases. N Engl J Med 2001;344:30-36.
9. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. J Allergy Clin Immunol 2001;108:S2-8.
10. van Cauwenberge P, et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000;55:116-134.
11. Ownby DR. Skin tests in comparison to other diagnostic methods. Immunol Allergy Clin North Am 2001;21(2):355-367.
12. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol 1999;103:773-779.
13. Bernstein IL, et al. Practice parameters for allergy diagnostic testing. Ann Allergy Asthma Immunol 1995;75:543-625.
14. Nelson HS, Lahr J, Buchmeier A, McCormick D. Evaluation of devices for skin prick testing. J Allergy Clin Immunol 1998;101:153-156.
15. Merrett TG, Pantin CF, Dimond AH, Merrett J. Screening for IgE-mediated allergy. Allergy 1980 Sep;35(6):491-501.
16. Ferguson BJ. Cost effective pharmacotherapy for allergic rhinitis. Otolaryngol Clin North Am 1998;31(1):91-110.
17. Thompson AK, Juniper E, Meltzer EO. Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol 2000;85:338-348.
18. Eggleston PA, Bush RK. Environmental allergen avoidance: an overview. J Allergy Clin Immunol 2001;107:S403-405.
19. Wood RA, Johnson EF, van Atta ML, et al. A placebo-controlled trial of a HEPA air filter in the treatment of cat allergy. Am J Resp CC Med 1998;158:115-120.
20. Kramer MS. Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk (Cochrane Review). In: The Cochrane Library, Issue 3, 2001. Oxford: Update software.
21. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
22. Condemi J, Schulz R, Lim J. Triamcinolone acetonide aqueous nasal spray versus loratadine in seasonal allergic rhinitis: efficacy and quality of life. Ann Allergy Asthma Immunol 2000;84:533-538.
23. Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract 1998;47:118-125.
24. Small P, et al. Comparison of triamcinalone acetonide nasal aerosol spray and fluticasone propionate aqueous solution spray in treatment of spring allergic rhinitis. J Allergy Clin Immunol 1997;100(5):592-5.
25. Day J, Carrillo T. Comparison of the efficacy of budesonide and fluticasone propionate aqueous nasal spray for once daily treatment of perennial allergic rhinitis. J Allergy Clin Immunol 1998;102:902-908.
26. Jen A, Baroody F, de Tineo M, et al. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 2000;105:732-738
27. Naclerio RM. Allergic rhinitis. N Engl J Med 1991;325:860-869.
28. Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol 2000;105:S622-627.
29. Abramowicz M. (Ed.) Newer antihistamines. Med Letter 2001;43:35.-
30. Bender BG, McCormick DR, Milgrom H. Children’s school performance is not impaired by short-term administration of diphenhydramine or loratadine. J Peds 2001;138:656-660.
31. Meltzer EO. Once-daily fexofenadine HCl improves quality of life and reduces work and activity impairment in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol 1999;83:311-317.
32. Sussman GL, Mason J, Compton D, Stewart J, Ricard N. The efficacy and safety of fexofenadine HCL and pseudophedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol 1999;104:100-106.
33. Graf P, Enerdal J, Hallen H. Ten days’use of oxymetazoline nasal spray with benzalkonioum chloride in patients with vasomotor rhinitis. Arch Otolaryngol Head and Neck Surg 1999;125:1128-1132.
34. Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: A randomized placebo-controlled clinical trial. J Allergy Clin Immunol 2000;105:917-922.
35. LaForce C. Use of nasal steroids in managing allergic rhinitis. J Allergy Clin Immunol 1999;103:S388-394.
36. Williams PV. Treatment of rhinitis: corticosteroids and cromolyn sodium. Immunol Allergy Clin North Am 2000;20:369-381.
37. Craig T, Sawyer AM, Fornadley JA. Use of immunotherapy in a primary care office. Am Fam Phys 1998;57:1888-1894.
38. Durham SR, Walker SM, Varga EM, et al. Long-term efficacy of grass-pollen immunotherapy. N Engl J Med 1999;341:468-475.
39. Bielory L, Lupoli K. Herbal interventions in asthma and allergy. J Asthma 1999;36(1):1-65.
40. Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076-1079.
41. Juniper EF, Guyatt GJ, Ferrie PJ, Griffith LE. First-line treatment of seasonal (ragweed) rhinoconjunctivitis. Can Med Assoc J 1997;156:1123-1131.
42. Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol 1997;99:S773-780.
43. Skoner DP. Complications of allergic rhinitis. J Allergy Clin Immunol 2000;105:S605-609.
44. Settipane RA. Complications of allergic rhinitis. Allergy Asthma Proc 1999;20(4):209-213.
45. Buck ML. Intranasal steroids for Children with Allergic Rhinitis. Pediatric Pharmacotherapy 2000;(7)5:1-12.
- Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”).
- Skin prick testing can detect IgE antibodies in patients with reliable histories of exposure to allergens.
- Intranasal corticosteroids are superior to other medications in achieving desired clinical outcomes, including quality of life.
- For some cases of allergic rhinitis, subcutaneous immunotherapy can achieve clinical remission for up to 3 years after cessation of therapy.
While allergic rhinitis is merely a nuisance to most people afflicted by it, the condition can lead to complications if it is severe or exists undetected for too long. In this article, I review the most reliable means of diagnosing allergic rhinitis, and outline a recommended approach to treatment.
Prevalence and pathophysiology
An estimated 20 to 40 million Americans are affected by allergic rhinitis. The actual prevalence of the condition is difficult to discern as many sufferers self-medicate without seeking medical care. One survey stated that up to 92% of patients had self-medicated prior to seeking medical care.1 Even when accounting for self-treatment, allergic rhinitis is the most commonly encountered form of chronic rhinitis, representing about 3% of all primary care office visits.2,3 Direct and indirect clinical costs run between $1.2 and $5.3 billion per year.4-6 Although the disease can develop in persons of any age, in 80% of cases symptoms will develop before the patient is 20 years old.5 Symptoms often wane as a patient grows older, and it is uncommon for persons older than 65 to experience new onset of allergic rhinitis.3,7
Allergic rhinitis stems from a type I hypersensitivity reaction.4 During an initial sensitization phase, the immune system identifies an allergen as foreign and generates specific antibodies to act against that allergen. Atopic patients exhibit an exaggerated response, generating high levels of Type 2 T-helper (Th2) cells and, subsequently, IgE antibodies.8 On reexposure to the allergen, specific IgE antibodies bound to mast cells form cross-links resulting in mast cell degranulation and the release of histamine and other chemical mediators. The patient then immediately develops such allergy symptoms as itching, sneezing, and rhinorrhea. A cellular inflammatory response, chiefly involving eosinophils, monocytes, and basophils, characterizes the secondary phase of the allergic reaction. Nasal congestion tends to dominate this later response phase.
Seasonal allergic rhinitis is usually triggered by pollens or molds. Perennial allergic rhinitis, triggered by dust mites, molds, cockroach or animal allergens, is defined as occurring 9 months out of the year.9
Clinical evaluation
The diagnosis of allergic rhinitis is usually made on the basis of the patient’s history and the results of your physical examination. In addition to classic symptoms of nasal congestion, itchy nose, sneezing, rhinorrhea, or itchy, watery eyes, patients may also complain of chronic cough, dry scratchy throat, otalgia, or recurrent sinusitis.4 Other important historical considerations include a family history of allergic rhinitis, a history of other atopic disease, previous treatment experiences, and suspected triggers.5
Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”). Chronic nasal congestion may also precipitate darkening of the skin under the eyes or “allergic shiners.”2,6 Concurrent conjunctivitis is common. Polyps, seen on direct nasal examination, may occur both in allergic and non-allergic patients.
No studies have evaluated the accuracy of the history or physical examination in confirming the diagnosis of allergic rhinitis. The differential diagnosis is extensive and includes infectious rhinitis, non-allergic rhinitis with eosinophilia syndrome (NARES), occupational rhinitis, mechanical obstruction, vasomotor rhinitis, drug-induced rhinitis, and nasal polyps.5
Diagnostic tests
Published guidelines from the American Academy of Asthma, Allergy and Immunology, as well as other expert panels, recommend confirmatory testing when allergic rhinitis is clinically suspected.2,5,10 There is no evidence to support the superiority of this recommendation over an empiric trial of medication, and most primary care physicians choose to treat empirically based upon the history and physical examination.
Although further testing should be done when the diagnosis is unclear, be aware that there is uncertainty associated with allergy testing. Because an individual may become sensitized to an allergen without exhibiting symptoms of allergic rhinitis, there is no clearly defined reference standard for the confirmation of allergic rhinitis.11 Likewise, a history of sensitivity is not always followed by expected IgE test results. Challenge methods developed for studies of airborne allergens are used as reference standards in the evaluation of clinical tests.12
Diagnostic tests include skin prick testing, intradermal testing, and in vitro blood tests. Nasal challenge testing, nasal smears, sinus transillumination, and nasopharyngoscopy are nonspecific tests. They are not recommended for routine evaluation but may be useful in selected cases when allergen-specific tests have failed to clarify the cause of the rhinitis. An expert panel has stated that no studies address the cost-effectiveness of any of these methods.2
Skin prick testing (SPT) is considered the most convenient and least expensive screening test. SPT can detect IgE antibodies in patients with reliable exposure histories.13 Sensitivity and specificity are difficult to determine, for a number of reasons. First, as previously mentioned, there is no clearly defined reference standard.11 Second, only 5 allergen extracts have been standardized for defined quantities known to induce biologic activity. Standardized extracts in the United States include ragweed pollen, cat dander, house dust mites, Hymenoptera venoms, and some grasses. All other extracts are local or regional preparations, and skin tests with nonstandard extracts are not necessarily reproducible.11 Third, even with a single individual, there can be wide variation in skin reaction to the same reagent, depending on the device used.14 As a result, correlation between SPT and inhalation challenges vary from 60% to 90%.13
Intradermal skin tests (IDST) are usually done when SPT yields a negative result despite a history compatible with allergic rhinitis.13 The primary advantage of IDST is sensitivity afforded by a fixed concentration of allergen. Because of this sensitivity, not all reactions are clinically relevant.13 In fact, IDST is often used as a reference standard in studies of the accuracy of SPT and in vitro tests.
Several in vitro assays of specific IgE antibodies are available. They are all modeled after the original radioallergosorbent tests (RAST); the term “RAST” is often used interchangeably with any type of in vitro blood test.13 IgE antibody tests have a high false-positive rate, meaning the test is positive in patients without allergy symptoms. RAST tests are less sensitive than SPT, with a mean sensitivity of 75% and a range of approximately 50% to 95%.13
The 3 primary diagnostic tests for allergic rhinitis are usually compared with each other and not to a recognized standard. Table 1 summarizes data from a study that compared all 3 tests with subjects who were placed in a small room with 2 cats and their bed.12 While this is one of very few studies that contrasts all 3 tests to a reasonable reference standard, the findings cannot necessarily be extrapolated to other airborne allergens.
In the hope of limiting referrals to allergists for testing, and reducing the uncertainty in making a diagnosis, one study looked at the RAST response to 19 allergens. The authors found that of all the patients who responded to any allergen, 95% exhibited responses specifically to grass pollen, dust mites, or cat dander. They went on to conclude that 96.3% of patients with allergic disease could be correctly identified with a combination of a standardized history (available in the study text), a total serum IgE of greater than 40 U/mL, and in vitro tests for cat dander, dust mites, and grass pollen.15
TABLE 1
Accuracy of diagnostic tests for diagnosis of cat allergy13
| Test | Sensitivity | Specificity | PV+ | PV- | LR+ | LR- |
|---|---|---|---|---|---|---|
| Skin prick test | 79.2 | 90.6 | 92.6 | 74.3 | 8.4 | 0.2 |
| Intradermal test | 60.0 | 31.0 | 23.1 | 69.2 | 0.9 | 1.3 |
| RAST | 69.2 | 100 | 100 | 72.7 | 69.2 | 0.3 |
| Note: Results are based upon any upper or lower symptoms when exposed to cat challenge. Intradermal test done if negative skin prick test. LR+ = positive likelihood ratio, LR- = negative likelihood ratio, PV+ = positive predictive value, PV- = negative predictive value. | ||||||
Treatment
Untreated allergic rhinitis can have a significant impact on quality of life. Patients are bothered by nose blowing, disrupted sleep, fatigue, and decreased concentration.1 In one 1996 survey, 32 % of patients said that allergy attacks embarrassed them or interfered with their quality of life.16 As a result, most patient-oriented studies on treatment evaluate the impact on health-related quality of life.17
The initial form of treatment is usually avoidance of the allergen, although this can be difficult. For animal allergens, washing pets and using high-efficiency particulate air (HEPA) filters have been shown to temporarily reduce the volume of airborne allergens but not to improve patient-oriented outcomes.18,19 Removing the pet from the home is the only sure remedy.18 More studies are needed to evaluate the benefit of multiple home treatments to reduce exposure to cockroach and fungal allergens.18 A systematic review of several studies showed that maternal antigen avoidance during lactation reduced the incidence of atopic dermatitis in at-risk infants.20 A meta-analysis of measures to avoid house-dust mites showed no clear benefit for patients with asthma10 It is unclear if these findings can be extrapolated to other atopic conditions such as allergic rhinitis.
Intranasal corticosteroids. Intranasal corticosteroids are the most effective medication in the treatment of allergic rhinitis. Available preparations in the US include beclomethasone diproprionate, budesonide, funisolide, fluticasone propionate, mometa-sone furoate, and triamcinalone acetonide. A meta-analysis identified 16 randomized controlled trials (RCTs) that compared antihistamines with intranasal corticosteroids in a total of 2767 patients. Intranasal corticosteroids provided significantly greater relief from nasal discharge, sneezing, pruritis, and postnasal drip. There was no statistically significant difference between the 2 in reduction of eye symptoms.21
Although this review did not address quality of life, other studies have shown that both triamcinolone acetonide and fluticasone propionate are superior to loratadine in improving quality of life.22,23 Few studies provide any guidance in choosing one intranasal steroid over another. Generally, they are of equal efficacy in patient-oriented outcomes.24,25 Although intranasal corticosteroids are considered daily or “maintenance” medications, a single small RCT of 26 patients showed that fluticasone propionate improved quality of life and reduced symptoms compared with placebo when used on an as-needed basis over a 4-week period.26 More studies are needed to confirm this preliminary finding, though.
Antihistamines. Although not as effective as intranasal steroids, antihistamines do reduce symptoms of rhinorrhea, sneezing, and itching.27 First-gen-eration antihistamines (diphenhydramine, chlorpheniramine, etc.) are lipophilic and cross the blood-brain barrier, resulting in varying degrees of anticholinergic side effects. Placebo-controlled studies have confirmed that these agents cause psychomotor retardation, sleepiness, and decreased work production.5,28 Specifically they seem to affect attention, memory, and vigilance. These symptoms may persist even after an overnight period of sleep.28,29 Second-generation antihistamines (fexofenadine, loratadine, etc.) do not penetrate the brain as well and are less likely to cause central nervous system effects.
However, a recent RCT involving 63 elementary school students challenges findings from previous studies. Children who received diphenhydramine, 25 mg twice daily, performed no differently on computerized reaction-time tests or multiple-choice learning tests than did children who received placebo or loratadine, 10 mg daily.30 Another RCT involving 845 patients from ages 12 to 65 years evaluated quality of life as well as work and school performance of patients who received fexofenadine or placebo. While quality-of-life scores and work performance improved significantly with fexofenadine, there was no significant difference between the groups in school performance.31 Direct comparisons of antihistamines are rare and the results are conflicting. There are no data to show that one of the first-generation antihistamines is superior to the others. Similarly, second-generation drugs are no more effective than the older medications; they only have fewer side effects. Among the second-generation antihistamines, fexofenadine and cetirizine appear to be more effective then loratadine.29
Decongestants. Systemic and topical decongestants relieve the congestion that accompanies the secondary phase of an allergic reaction.4 They have limited effects on other allergic symptoms and, as a result, are often used in combination with antihistamines.32 When used for more than 10 days, topical decongestants (oxymetazoline, xylometazoline) are associated with rebound congestion (rhinitis medicamentosa).33
Leukotriene receptor antagonists. Although not approved by the FDA for treatment of allergic rhinitis, the leukotriene receptor antagonist montelukast was shown in a randomized double-blinded trial to be as effective as loratadine in relieving symptoms. There was minimal additional benefit in using the medications concomitantly.34
Cromolyn sodium. Cromolyn sodium has been shown to prevent the onset of allergic rhinitis symptoms in multiple placebo-controlled trials.35 It is extremely safe but requires regular use and is not as effective as other medications for acute symptoms. Direct comparison studies have shown that cromolyn is not as effective as intranasal corticosteroids.35,36
Immunotherapy. Subcutaneous immunotherapy (SIT) is recommended by all guidelines for patients who fail to respond to pharmacotherapy and allergen avoidance.2,5,10,27 It is recommended in particular for allergic rhinitis secondary to ragweed, grasses, molds, and dust mites. Immunotherapy induces the creation of protective IgG and inhibits the inflammatory response to allergens.27 SIT requires specific allergen confirmation with either a skin test or in vitro assay. Preparation of SIT doses should be done by a practitioner well trained in mixing and diluting extracts.5,37 Forty-three placebo-controlled, double-blind studies have evaluated the efficacy of SIT for 12 different allergens since 1980.10 Thirty-two trials showed clinical efficacy, which can be long lasting. A study of patients treated for 3 to 4 years with immunotherapy for grass pollen allergy showed continued clinical remission for at least 3 years after treatment was stopped.38
Herbal therapies. Alternative approaches to the treatment of allergic rhinitis warrant further investigation. Herbal medications, such as licorice, gingko, and ginseng, are currently used to treat allergic rhinitis, although there are no large studies to confirm their effectiveness.39
Probiotics. Epidemiologic studies suggest that the increase in atopic disease may be related to a clean environment and widespread use of antibiotics in Western countries. The environment may deprive fetal and infant immune systems of bacterial antigens that stimulate type 1 T-helper (Th1) cells.8 In light of this theory, Finnish researchers randomly assigned 159 pregnant women with a family history of atopy to receive capsules of Lactobacillus GG (a potentially beneficial bacteria or “probiotic”) or placebo, beginning 2 to 4 weeks prior to delivery and continuing 6 months postpartum. Infants were followed for 2 years. Frequency of atopic dermatitis was reduced by 50% among those infants whose mothers received Lactobacillus.40 Further study of this association in allergic rhinitis would be beneficial.
Treatment recommendations.Table 2 summarizes treatment-related evidence in the management of allergic rhinitis, and the Figure illustrates a proposed treatment algorithm. Another algorithm by the European Academy of Allergy and Clinical Immunology recommends initial therapy with oral or nasal antihistamines for mild disease, nasal corticosteroids for moderate disease, and both for severe disease.10 This was a consensus opinion. Of the 2 most commonly used medications for allergy, nasal steroids are favored over antihistamines for overall safety, tolerability, effectiveness, and simplicity in all cases. In one study of 61 adults, patients were randomized to receive either a nasal steroid or an antihistamine as initial therapy, with the other agent reserved as “back-up.” After 6 weeks, 86% of patients started on an antihistamine had added their steroid back-up, while 51% of the group started on a steroid remained on that agent alone.41 Starting all patients on both an antihistamine and a nasal steroid is inappropriate.
TABLE 2
Evidence to support treatment recommendations
| Strength of recommendation | Treatment | Comment |
|---|---|---|
| A | Immunotherapy | Can have long lasting clinical benefit. |
| A | Intranasal Consistently | superior to antihistamines corticosteroids in head to head trials. Not clear if all steroids are equally effective. |
| A | Antihistamines | Effective, but inferior to intranasal steroids in most clinical outcomes. |
| A | Cromolyn sodium | Intranasal steroids superior in all clinical outcomes. |
| B | Decongestants | Less effective than antihistamines in direct comparisons, many trials involve combination products. |
| D | Probiotics | Larger trials needed, limited evidence. |
| D | Herbal medications (licorice, gingko, ginseng) | Limited evidence. |
FIGUREA guide to evaluation and treatment of allergic rhinitis
Prognosis
The long-term prognosis for allergic rhinitis is excellent. For most patients, the illness is primarily a nuisance with no significant morbidity. However, for patients whose rhinitis is moderate to severe and poorly controlled, there can be significant complications. These complications include asthma, sinusitis, otitis media, nasal polyposis, respiratory infections, and orthodontic malocclusions.42 In one study of 605 children with allergic rhinitis, 21% had chronic otitis media with effusion (OME). Conversely, in another study of 259 children with OME, 50% had allergic rhinitis.43 Even among patients without asthma, 20% to 30% will have bronchial hyper-responsiveness. Additionally, poorly controlled allergic rhinitis can contribute to sleep loss, daytime fatigue, and learning impairment.44
Potential complications related to long-term treatment in children remain controversial. In a 1998 study of intranasal beclomethasone, children receiving the study medication grew an average of only 5 centimeters (cm) in 1 year, compared with an average of 5.9 cm in the placebo group.45 However, a similar study done 2 years later with intranasal mometa-sone showed no evidence of growth suppression.45 Further studies are needed before the true impact of intranasal steroids on children can be determined.
· Acknowledgments ·
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. The author wishes to thank Kathleen Conner, JD, for her assistance in the preparation of the manuscript.
- Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”).
- Skin prick testing can detect IgE antibodies in patients with reliable histories of exposure to allergens.
- Intranasal corticosteroids are superior to other medications in achieving desired clinical outcomes, including quality of life.
- For some cases of allergic rhinitis, subcutaneous immunotherapy can achieve clinical remission for up to 3 years after cessation of therapy.
While allergic rhinitis is merely a nuisance to most people afflicted by it, the condition can lead to complications if it is severe or exists undetected for too long. In this article, I review the most reliable means of diagnosing allergic rhinitis, and outline a recommended approach to treatment.
Prevalence and pathophysiology
An estimated 20 to 40 million Americans are affected by allergic rhinitis. The actual prevalence of the condition is difficult to discern as many sufferers self-medicate without seeking medical care. One survey stated that up to 92% of patients had self-medicated prior to seeking medical care.1 Even when accounting for self-treatment, allergic rhinitis is the most commonly encountered form of chronic rhinitis, representing about 3% of all primary care office visits.2,3 Direct and indirect clinical costs run between $1.2 and $5.3 billion per year.4-6 Although the disease can develop in persons of any age, in 80% of cases symptoms will develop before the patient is 20 years old.5 Symptoms often wane as a patient grows older, and it is uncommon for persons older than 65 to experience new onset of allergic rhinitis.3,7
Allergic rhinitis stems from a type I hypersensitivity reaction.4 During an initial sensitization phase, the immune system identifies an allergen as foreign and generates specific antibodies to act against that allergen. Atopic patients exhibit an exaggerated response, generating high levels of Type 2 T-helper (Th2) cells and, subsequently, IgE antibodies.8 On reexposure to the allergen, specific IgE antibodies bound to mast cells form cross-links resulting in mast cell degranulation and the release of histamine and other chemical mediators. The patient then immediately develops such allergy symptoms as itching, sneezing, and rhinorrhea. A cellular inflammatory response, chiefly involving eosinophils, monocytes, and basophils, characterizes the secondary phase of the allergic reaction. Nasal congestion tends to dominate this later response phase.
Seasonal allergic rhinitis is usually triggered by pollens or molds. Perennial allergic rhinitis, triggered by dust mites, molds, cockroach or animal allergens, is defined as occurring 9 months out of the year.9
Clinical evaluation
The diagnosis of allergic rhinitis is usually made on the basis of the patient’s history and the results of your physical examination. In addition to classic symptoms of nasal congestion, itchy nose, sneezing, rhinorrhea, or itchy, watery eyes, patients may also complain of chronic cough, dry scratchy throat, otalgia, or recurrent sinusitis.4 Other important historical considerations include a family history of allergic rhinitis, a history of other atopic disease, previous treatment experiences, and suspected triggers.5
Physical clues to allergic rhinitis include boggy, pale, or “bluish” nasal turbinates, with watery discharge on nasal speculum exam. Patients may also have a nasal crease on the external nose caused by repeated rubbing or itching (the so-called “allergic salute”). Chronic nasal congestion may also precipitate darkening of the skin under the eyes or “allergic shiners.”2,6 Concurrent conjunctivitis is common. Polyps, seen on direct nasal examination, may occur both in allergic and non-allergic patients.
No studies have evaluated the accuracy of the history or physical examination in confirming the diagnosis of allergic rhinitis. The differential diagnosis is extensive and includes infectious rhinitis, non-allergic rhinitis with eosinophilia syndrome (NARES), occupational rhinitis, mechanical obstruction, vasomotor rhinitis, drug-induced rhinitis, and nasal polyps.5
Diagnostic tests
Published guidelines from the American Academy of Asthma, Allergy and Immunology, as well as other expert panels, recommend confirmatory testing when allergic rhinitis is clinically suspected.2,5,10 There is no evidence to support the superiority of this recommendation over an empiric trial of medication, and most primary care physicians choose to treat empirically based upon the history and physical examination.
Although further testing should be done when the diagnosis is unclear, be aware that there is uncertainty associated with allergy testing. Because an individual may become sensitized to an allergen without exhibiting symptoms of allergic rhinitis, there is no clearly defined reference standard for the confirmation of allergic rhinitis.11 Likewise, a history of sensitivity is not always followed by expected IgE test results. Challenge methods developed for studies of airborne allergens are used as reference standards in the evaluation of clinical tests.12
Diagnostic tests include skin prick testing, intradermal testing, and in vitro blood tests. Nasal challenge testing, nasal smears, sinus transillumination, and nasopharyngoscopy are nonspecific tests. They are not recommended for routine evaluation but may be useful in selected cases when allergen-specific tests have failed to clarify the cause of the rhinitis. An expert panel has stated that no studies address the cost-effectiveness of any of these methods.2
Skin prick testing (SPT) is considered the most convenient and least expensive screening test. SPT can detect IgE antibodies in patients with reliable exposure histories.13 Sensitivity and specificity are difficult to determine, for a number of reasons. First, as previously mentioned, there is no clearly defined reference standard.11 Second, only 5 allergen extracts have been standardized for defined quantities known to induce biologic activity. Standardized extracts in the United States include ragweed pollen, cat dander, house dust mites, Hymenoptera venoms, and some grasses. All other extracts are local or regional preparations, and skin tests with nonstandard extracts are not necessarily reproducible.11 Third, even with a single individual, there can be wide variation in skin reaction to the same reagent, depending on the device used.14 As a result, correlation between SPT and inhalation challenges vary from 60% to 90%.13
Intradermal skin tests (IDST) are usually done when SPT yields a negative result despite a history compatible with allergic rhinitis.13 The primary advantage of IDST is sensitivity afforded by a fixed concentration of allergen. Because of this sensitivity, not all reactions are clinically relevant.13 In fact, IDST is often used as a reference standard in studies of the accuracy of SPT and in vitro tests.
Several in vitro assays of specific IgE antibodies are available. They are all modeled after the original radioallergosorbent tests (RAST); the term “RAST” is often used interchangeably with any type of in vitro blood test.13 IgE antibody tests have a high false-positive rate, meaning the test is positive in patients without allergy symptoms. RAST tests are less sensitive than SPT, with a mean sensitivity of 75% and a range of approximately 50% to 95%.13
The 3 primary diagnostic tests for allergic rhinitis are usually compared with each other and not to a recognized standard. Table 1 summarizes data from a study that compared all 3 tests with subjects who were placed in a small room with 2 cats and their bed.12 While this is one of very few studies that contrasts all 3 tests to a reasonable reference standard, the findings cannot necessarily be extrapolated to other airborne allergens.
In the hope of limiting referrals to allergists for testing, and reducing the uncertainty in making a diagnosis, one study looked at the RAST response to 19 allergens. The authors found that of all the patients who responded to any allergen, 95% exhibited responses specifically to grass pollen, dust mites, or cat dander. They went on to conclude that 96.3% of patients with allergic disease could be correctly identified with a combination of a standardized history (available in the study text), a total serum IgE of greater than 40 U/mL, and in vitro tests for cat dander, dust mites, and grass pollen.15
TABLE 1
Accuracy of diagnostic tests for diagnosis of cat allergy13
| Test | Sensitivity | Specificity | PV+ | PV- | LR+ | LR- |
|---|---|---|---|---|---|---|
| Skin prick test | 79.2 | 90.6 | 92.6 | 74.3 | 8.4 | 0.2 |
| Intradermal test | 60.0 | 31.0 | 23.1 | 69.2 | 0.9 | 1.3 |
| RAST | 69.2 | 100 | 100 | 72.7 | 69.2 | 0.3 |
| Note: Results are based upon any upper or lower symptoms when exposed to cat challenge. Intradermal test done if negative skin prick test. LR+ = positive likelihood ratio, LR- = negative likelihood ratio, PV+ = positive predictive value, PV- = negative predictive value. | ||||||
Treatment
Untreated allergic rhinitis can have a significant impact on quality of life. Patients are bothered by nose blowing, disrupted sleep, fatigue, and decreased concentration.1 In one 1996 survey, 32 % of patients said that allergy attacks embarrassed them or interfered with their quality of life.16 As a result, most patient-oriented studies on treatment evaluate the impact on health-related quality of life.17
The initial form of treatment is usually avoidance of the allergen, although this can be difficult. For animal allergens, washing pets and using high-efficiency particulate air (HEPA) filters have been shown to temporarily reduce the volume of airborne allergens but not to improve patient-oriented outcomes.18,19 Removing the pet from the home is the only sure remedy.18 More studies are needed to evaluate the benefit of multiple home treatments to reduce exposure to cockroach and fungal allergens.18 A systematic review of several studies showed that maternal antigen avoidance during lactation reduced the incidence of atopic dermatitis in at-risk infants.20 A meta-analysis of measures to avoid house-dust mites showed no clear benefit for patients with asthma10 It is unclear if these findings can be extrapolated to other atopic conditions such as allergic rhinitis.
Intranasal corticosteroids. Intranasal corticosteroids are the most effective medication in the treatment of allergic rhinitis. Available preparations in the US include beclomethasone diproprionate, budesonide, funisolide, fluticasone propionate, mometa-sone furoate, and triamcinalone acetonide. A meta-analysis identified 16 randomized controlled trials (RCTs) that compared antihistamines with intranasal corticosteroids in a total of 2767 patients. Intranasal corticosteroids provided significantly greater relief from nasal discharge, sneezing, pruritis, and postnasal drip. There was no statistically significant difference between the 2 in reduction of eye symptoms.21
Although this review did not address quality of life, other studies have shown that both triamcinolone acetonide and fluticasone propionate are superior to loratadine in improving quality of life.22,23 Few studies provide any guidance in choosing one intranasal steroid over another. Generally, they are of equal efficacy in patient-oriented outcomes.24,25 Although intranasal corticosteroids are considered daily or “maintenance” medications, a single small RCT of 26 patients showed that fluticasone propionate improved quality of life and reduced symptoms compared with placebo when used on an as-needed basis over a 4-week period.26 More studies are needed to confirm this preliminary finding, though.
Antihistamines. Although not as effective as intranasal steroids, antihistamines do reduce symptoms of rhinorrhea, sneezing, and itching.27 First-gen-eration antihistamines (diphenhydramine, chlorpheniramine, etc.) are lipophilic and cross the blood-brain barrier, resulting in varying degrees of anticholinergic side effects. Placebo-controlled studies have confirmed that these agents cause psychomotor retardation, sleepiness, and decreased work production.5,28 Specifically they seem to affect attention, memory, and vigilance. These symptoms may persist even after an overnight period of sleep.28,29 Second-generation antihistamines (fexofenadine, loratadine, etc.) do not penetrate the brain as well and are less likely to cause central nervous system effects.
However, a recent RCT involving 63 elementary school students challenges findings from previous studies. Children who received diphenhydramine, 25 mg twice daily, performed no differently on computerized reaction-time tests or multiple-choice learning tests than did children who received placebo or loratadine, 10 mg daily.30 Another RCT involving 845 patients from ages 12 to 65 years evaluated quality of life as well as work and school performance of patients who received fexofenadine or placebo. While quality-of-life scores and work performance improved significantly with fexofenadine, there was no significant difference between the groups in school performance.31 Direct comparisons of antihistamines are rare and the results are conflicting. There are no data to show that one of the first-generation antihistamines is superior to the others. Similarly, second-generation drugs are no more effective than the older medications; they only have fewer side effects. Among the second-generation antihistamines, fexofenadine and cetirizine appear to be more effective then loratadine.29
Decongestants. Systemic and topical decongestants relieve the congestion that accompanies the secondary phase of an allergic reaction.4 They have limited effects on other allergic symptoms and, as a result, are often used in combination with antihistamines.32 When used for more than 10 days, topical decongestants (oxymetazoline, xylometazoline) are associated with rebound congestion (rhinitis medicamentosa).33
Leukotriene receptor antagonists. Although not approved by the FDA for treatment of allergic rhinitis, the leukotriene receptor antagonist montelukast was shown in a randomized double-blinded trial to be as effective as loratadine in relieving symptoms. There was minimal additional benefit in using the medications concomitantly.34
Cromolyn sodium. Cromolyn sodium has been shown to prevent the onset of allergic rhinitis symptoms in multiple placebo-controlled trials.35 It is extremely safe but requires regular use and is not as effective as other medications for acute symptoms. Direct comparison studies have shown that cromolyn is not as effective as intranasal corticosteroids.35,36
Immunotherapy. Subcutaneous immunotherapy (SIT) is recommended by all guidelines for patients who fail to respond to pharmacotherapy and allergen avoidance.2,5,10,27 It is recommended in particular for allergic rhinitis secondary to ragweed, grasses, molds, and dust mites. Immunotherapy induces the creation of protective IgG and inhibits the inflammatory response to allergens.27 SIT requires specific allergen confirmation with either a skin test or in vitro assay. Preparation of SIT doses should be done by a practitioner well trained in mixing and diluting extracts.5,37 Forty-three placebo-controlled, double-blind studies have evaluated the efficacy of SIT for 12 different allergens since 1980.10 Thirty-two trials showed clinical efficacy, which can be long lasting. A study of patients treated for 3 to 4 years with immunotherapy for grass pollen allergy showed continued clinical remission for at least 3 years after treatment was stopped.38
Herbal therapies. Alternative approaches to the treatment of allergic rhinitis warrant further investigation. Herbal medications, such as licorice, gingko, and ginseng, are currently used to treat allergic rhinitis, although there are no large studies to confirm their effectiveness.39
Probiotics. Epidemiologic studies suggest that the increase in atopic disease may be related to a clean environment and widespread use of antibiotics in Western countries. The environment may deprive fetal and infant immune systems of bacterial antigens that stimulate type 1 T-helper (Th1) cells.8 In light of this theory, Finnish researchers randomly assigned 159 pregnant women with a family history of atopy to receive capsules of Lactobacillus GG (a potentially beneficial bacteria or “probiotic”) or placebo, beginning 2 to 4 weeks prior to delivery and continuing 6 months postpartum. Infants were followed for 2 years. Frequency of atopic dermatitis was reduced by 50% among those infants whose mothers received Lactobacillus.40 Further study of this association in allergic rhinitis would be beneficial.
Treatment recommendations.Table 2 summarizes treatment-related evidence in the management of allergic rhinitis, and the Figure illustrates a proposed treatment algorithm. Another algorithm by the European Academy of Allergy and Clinical Immunology recommends initial therapy with oral or nasal antihistamines for mild disease, nasal corticosteroids for moderate disease, and both for severe disease.10 This was a consensus opinion. Of the 2 most commonly used medications for allergy, nasal steroids are favored over antihistamines for overall safety, tolerability, effectiveness, and simplicity in all cases. In one study of 61 adults, patients were randomized to receive either a nasal steroid or an antihistamine as initial therapy, with the other agent reserved as “back-up.” After 6 weeks, 86% of patients started on an antihistamine had added their steroid back-up, while 51% of the group started on a steroid remained on that agent alone.41 Starting all patients on both an antihistamine and a nasal steroid is inappropriate.
TABLE 2
Evidence to support treatment recommendations
| Strength of recommendation | Treatment | Comment |
|---|---|---|
| A | Immunotherapy | Can have long lasting clinical benefit. |
| A | Intranasal Consistently | superior to antihistamines corticosteroids in head to head trials. Not clear if all steroids are equally effective. |
| A | Antihistamines | Effective, but inferior to intranasal steroids in most clinical outcomes. |
| A | Cromolyn sodium | Intranasal steroids superior in all clinical outcomes. |
| B | Decongestants | Less effective than antihistamines in direct comparisons, many trials involve combination products. |
| D | Probiotics | Larger trials needed, limited evidence. |
| D | Herbal medications (licorice, gingko, ginseng) | Limited evidence. |
FIGUREA guide to evaluation and treatment of allergic rhinitis
Prognosis
The long-term prognosis for allergic rhinitis is excellent. For most patients, the illness is primarily a nuisance with no significant morbidity. However, for patients whose rhinitis is moderate to severe and poorly controlled, there can be significant complications. These complications include asthma, sinusitis, otitis media, nasal polyposis, respiratory infections, and orthodontic malocclusions.42 In one study of 605 children with allergic rhinitis, 21% had chronic otitis media with effusion (OME). Conversely, in another study of 259 children with OME, 50% had allergic rhinitis.43 Even among patients without asthma, 20% to 30% will have bronchial hyper-responsiveness. Additionally, poorly controlled allergic rhinitis can contribute to sleep loss, daytime fatigue, and learning impairment.44
Potential complications related to long-term treatment in children remain controversial. In a 1998 study of intranasal beclomethasone, children receiving the study medication grew an average of only 5 centimeters (cm) in 1 year, compared with an average of 5.9 cm in the placebo group.45 However, a similar study done 2 years later with intranasal mometa-sone showed no evidence of growth suppression.45 Further studies are needed before the true impact of intranasal steroids on children can be determined.
· Acknowledgments ·
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. The author wishes to thank Kathleen Conner, JD, for her assistance in the preparation of the manuscript.
1. Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S805-28.
2. Fornadley JA, et al. Allergic rhinitis: Clinical practice guideline. Otolaryngol Head Neck Surg 1996;115(1):115-122.
3. Wagner W, Kavuru M. Diagnosis and Management of Rhinitis. 1st Ed. Professional Communications, Inc. 1996. Available at www.medscape.com. Accessed Sept. 6, 2001.
4. Hadley JA. Evaluation and management of allergic rhinitis. Med Clin North Am 1999;83(1):13-25.
5. Dykewicz MS, Fineman S, et al. Diagnosis and management of rhinitis: complete guidelines of the joint task force on practice parameters in allergy, asthma and immunology. Ann Allergy Asthma Immunol 1998;81:478-518.
6. Weiss KB. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001;107:3-8.
7. Gentile DA, Friday GA, Skoner DP. Management of allergic rhinitis: antihistamines and decongestants. Immunol Allergy Clin North Am 2000;20(2):355-368.
8. Kay AB. Allergy and allergic diseases. N Engl J Med 2001;344:30-36.
9. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. J Allergy Clin Immunol 2001;108:S2-8.
10. van Cauwenberge P, et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000;55:116-134.
11. Ownby DR. Skin tests in comparison to other diagnostic methods. Immunol Allergy Clin North Am 2001;21(2):355-367.
12. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol 1999;103:773-779.
13. Bernstein IL, et al. Practice parameters for allergy diagnostic testing. Ann Allergy Asthma Immunol 1995;75:543-625.
14. Nelson HS, Lahr J, Buchmeier A, McCormick D. Evaluation of devices for skin prick testing. J Allergy Clin Immunol 1998;101:153-156.
15. Merrett TG, Pantin CF, Dimond AH, Merrett J. Screening for IgE-mediated allergy. Allergy 1980 Sep;35(6):491-501.
16. Ferguson BJ. Cost effective pharmacotherapy for allergic rhinitis. Otolaryngol Clin North Am 1998;31(1):91-110.
17. Thompson AK, Juniper E, Meltzer EO. Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol 2000;85:338-348.
18. Eggleston PA, Bush RK. Environmental allergen avoidance: an overview. J Allergy Clin Immunol 2001;107:S403-405.
19. Wood RA, Johnson EF, van Atta ML, et al. A placebo-controlled trial of a HEPA air filter in the treatment of cat allergy. Am J Resp CC Med 1998;158:115-120.
20. Kramer MS. Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk (Cochrane Review). In: The Cochrane Library, Issue 3, 2001. Oxford: Update software.
21. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
22. Condemi J, Schulz R, Lim J. Triamcinolone acetonide aqueous nasal spray versus loratadine in seasonal allergic rhinitis: efficacy and quality of life. Ann Allergy Asthma Immunol 2000;84:533-538.
23. Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract 1998;47:118-125.
24. Small P, et al. Comparison of triamcinalone acetonide nasal aerosol spray and fluticasone propionate aqueous solution spray in treatment of spring allergic rhinitis. J Allergy Clin Immunol 1997;100(5):592-5.
25. Day J, Carrillo T. Comparison of the efficacy of budesonide and fluticasone propionate aqueous nasal spray for once daily treatment of perennial allergic rhinitis. J Allergy Clin Immunol 1998;102:902-908.
26. Jen A, Baroody F, de Tineo M, et al. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 2000;105:732-738
27. Naclerio RM. Allergic rhinitis. N Engl J Med 1991;325:860-869.
28. Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol 2000;105:S622-627.
29. Abramowicz M. (Ed.) Newer antihistamines. Med Letter 2001;43:35.-
30. Bender BG, McCormick DR, Milgrom H. Children’s school performance is not impaired by short-term administration of diphenhydramine or loratadine. J Peds 2001;138:656-660.
31. Meltzer EO. Once-daily fexofenadine HCl improves quality of life and reduces work and activity impairment in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol 1999;83:311-317.
32. Sussman GL, Mason J, Compton D, Stewart J, Ricard N. The efficacy and safety of fexofenadine HCL and pseudophedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol 1999;104:100-106.
33. Graf P, Enerdal J, Hallen H. Ten days’use of oxymetazoline nasal spray with benzalkonioum chloride in patients with vasomotor rhinitis. Arch Otolaryngol Head and Neck Surg 1999;125:1128-1132.
34. Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: A randomized placebo-controlled clinical trial. J Allergy Clin Immunol 2000;105:917-922.
35. LaForce C. Use of nasal steroids in managing allergic rhinitis. J Allergy Clin Immunol 1999;103:S388-394.
36. Williams PV. Treatment of rhinitis: corticosteroids and cromolyn sodium. Immunol Allergy Clin North Am 2000;20:369-381.
37. Craig T, Sawyer AM, Fornadley JA. Use of immunotherapy in a primary care office. Am Fam Phys 1998;57:1888-1894.
38. Durham SR, Walker SM, Varga EM, et al. Long-term efficacy of grass-pollen immunotherapy. N Engl J Med 1999;341:468-475.
39. Bielory L, Lupoli K. Herbal interventions in asthma and allergy. J Asthma 1999;36(1):1-65.
40. Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076-1079.
41. Juniper EF, Guyatt GJ, Ferrie PJ, Griffith LE. First-line treatment of seasonal (ragweed) rhinoconjunctivitis. Can Med Assoc J 1997;156:1123-1131.
42. Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol 1997;99:S773-780.
43. Skoner DP. Complications of allergic rhinitis. J Allergy Clin Immunol 2000;105:S605-609.
44. Settipane RA. Complications of allergic rhinitis. Allergy Asthma Proc 1999;20(4):209-213.
45. Buck ML. Intranasal steroids for Children with Allergic Rhinitis. Pediatric Pharmacotherapy 2000;(7)5:1-12.
1. Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S805-28.
2. Fornadley JA, et al. Allergic rhinitis: Clinical practice guideline. Otolaryngol Head Neck Surg 1996;115(1):115-122.
3. Wagner W, Kavuru M. Diagnosis and Management of Rhinitis. 1st Ed. Professional Communications, Inc. 1996. Available at www.medscape.com. Accessed Sept. 6, 2001.
4. Hadley JA. Evaluation and management of allergic rhinitis. Med Clin North Am 1999;83(1):13-25.
5. Dykewicz MS, Fineman S, et al. Diagnosis and management of rhinitis: complete guidelines of the joint task force on practice parameters in allergy, asthma and immunology. Ann Allergy Asthma Immunol 1998;81:478-518.
6. Weiss KB. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001;107:3-8.
7. Gentile DA, Friday GA, Skoner DP. Management of allergic rhinitis: antihistamines and decongestants. Immunol Allergy Clin North Am 2000;20(2):355-368.
8. Kay AB. Allergy and allergic diseases. N Engl J Med 2001;344:30-36.
9. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. J Allergy Clin Immunol 2001;108:S2-8.
10. van Cauwenberge P, et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000;55:116-134.
11. Ownby DR. Skin tests in comparison to other diagnostic methods. Immunol Allergy Clin North Am 2001;21(2):355-367.
12. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol 1999;103:773-779.
13. Bernstein IL, et al. Practice parameters for allergy diagnostic testing. Ann Allergy Asthma Immunol 1995;75:543-625.
14. Nelson HS, Lahr J, Buchmeier A, McCormick D. Evaluation of devices for skin prick testing. J Allergy Clin Immunol 1998;101:153-156.
15. Merrett TG, Pantin CF, Dimond AH, Merrett J. Screening for IgE-mediated allergy. Allergy 1980 Sep;35(6):491-501.
16. Ferguson BJ. Cost effective pharmacotherapy for allergic rhinitis. Otolaryngol Clin North Am 1998;31(1):91-110.
17. Thompson AK, Juniper E, Meltzer EO. Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol 2000;85:338-348.
18. Eggleston PA, Bush RK. Environmental allergen avoidance: an overview. J Allergy Clin Immunol 2001;107:S403-405.
19. Wood RA, Johnson EF, van Atta ML, et al. A placebo-controlled trial of a HEPA air filter in the treatment of cat allergy. Am J Resp CC Med 1998;158:115-120.
20. Kramer MS. Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk (Cochrane Review). In: The Cochrane Library, Issue 3, 2001. Oxford: Update software.
21. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
22. Condemi J, Schulz R, Lim J. Triamcinolone acetonide aqueous nasal spray versus loratadine in seasonal allergic rhinitis: efficacy and quality of life. Ann Allergy Asthma Immunol 2000;84:533-538.
23. Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract 1998;47:118-125.
24. Small P, et al. Comparison of triamcinalone acetonide nasal aerosol spray and fluticasone propionate aqueous solution spray in treatment of spring allergic rhinitis. J Allergy Clin Immunol 1997;100(5):592-5.
25. Day J, Carrillo T. Comparison of the efficacy of budesonide and fluticasone propionate aqueous nasal spray for once daily treatment of perennial allergic rhinitis. J Allergy Clin Immunol 1998;102:902-908.
26. Jen A, Baroody F, de Tineo M, et al. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 2000;105:732-738
27. Naclerio RM. Allergic rhinitis. N Engl J Med 1991;325:860-869.
28. Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol 2000;105:S622-627.
29. Abramowicz M. (Ed.) Newer antihistamines. Med Letter 2001;43:35.-
30. Bender BG, McCormick DR, Milgrom H. Children’s school performance is not impaired by short-term administration of diphenhydramine or loratadine. J Peds 2001;138:656-660.
31. Meltzer EO. Once-daily fexofenadine HCl improves quality of life and reduces work and activity impairment in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol 1999;83:311-317.
32. Sussman GL, Mason J, Compton D, Stewart J, Ricard N. The efficacy and safety of fexofenadine HCL and pseudophedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol 1999;104:100-106.
33. Graf P, Enerdal J, Hallen H. Ten days’use of oxymetazoline nasal spray with benzalkonioum chloride in patients with vasomotor rhinitis. Arch Otolaryngol Head and Neck Surg 1999;125:1128-1132.
34. Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: A randomized placebo-controlled clinical trial. J Allergy Clin Immunol 2000;105:917-922.
35. LaForce C. Use of nasal steroids in managing allergic rhinitis. J Allergy Clin Immunol 1999;103:S388-394.
36. Williams PV. Treatment of rhinitis: corticosteroids and cromolyn sodium. Immunol Allergy Clin North Am 2000;20:369-381.
37. Craig T, Sawyer AM, Fornadley JA. Use of immunotherapy in a primary care office. Am Fam Phys 1998;57:1888-1894.
38. Durham SR, Walker SM, Varga EM, et al. Long-term efficacy of grass-pollen immunotherapy. N Engl J Med 1999;341:468-475.
39. Bielory L, Lupoli K. Herbal interventions in asthma and allergy. J Asthma 1999;36(1):1-65.
40. Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076-1079.
41. Juniper EF, Guyatt GJ, Ferrie PJ, Griffith LE. First-line treatment of seasonal (ragweed) rhinoconjunctivitis. Can Med Assoc J 1997;156:1123-1131.
42. Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol 1997;99:S773-780.
43. Skoner DP. Complications of allergic rhinitis. J Allergy Clin Immunol 2000;105:S605-609.
44. Settipane RA. Complications of allergic rhinitis. Allergy Asthma Proc 1999;20(4):209-213.
45. Buck ML. Intranasal steroids for Children with Allergic Rhinitis. Pediatric Pharmacotherapy 2000;(7)5:1-12.