User login
Brexanolone injection for postpartum depression
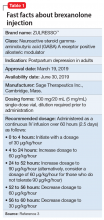
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
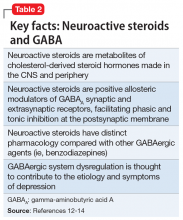
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
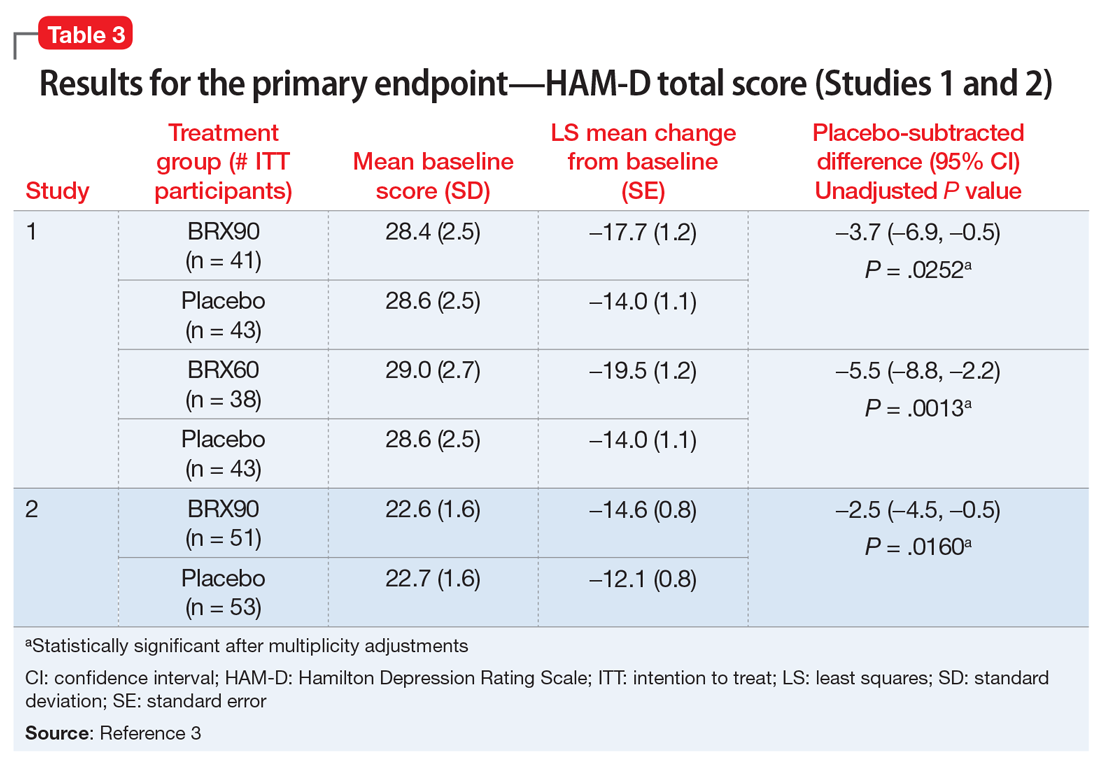
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
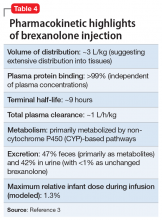
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.