User login
Woman With Blue-Gray Palate and Nail Beds
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
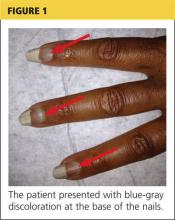
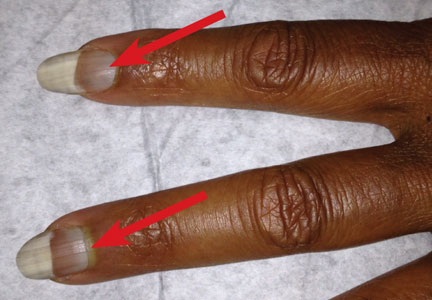
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
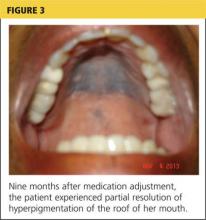
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.