User login
‘Joker’ filled with mental illness misconceptions
The Batman characters have been cultural icons for generations – spanning more than three-quarters of a century. How many of us had Batman (or the Joker) on our school lunch box or watched reruns of Adam West’s campy televised rendition of Batman? The October release of “Joker” has been breaking contemporary box office records.
(Spoiler alert!) The Todd Phillips film associates mental illness with violent acts, spurring a slew of articles explaining that this association is uncommon and may promote stigmatization and public fear of people with obvious symptoms of mental illness. The protagonist, Arthur Fleck (Joaquin Phoenix), suffers from a condition in which his affect and facial expressions are not appropriate to his emotions or to the situation. He laughs uncontrollably when a situation is sad or upsetting. Sometimes he laughs and cries at the same time. As a result, he often is misunderstood, ridiculed, and victimized – like many people with obvious mental illness.
Arthur Fleck is a loner who has difficulty with relationships and self-esteem, and is beaten severely while at work as a clown. Shortly after the incident, he is given a gun by one of his coworkers. He keeps it with him even when working as a clown in a children’s hospital – where it is accidentally revealed, and he is subsequently fired. Still in his clown garb, he later uses the gun when he is mocked and assaulted on the subway by three Gotham City bankers.
In an unusual tone, his mental health worker reminds him early in the film that he is prescribed seven different psychotropic medications, helping to cement for the viewer that mental illness is the cause of Arthur’s problems and the Joker’s origin story. Then the funding for Arthur’s mental health treatment (even if it was not good treatment) was cut – a problem not just in Gotham.
While some of Arthur Fleck’s symptoms are consistent with real mental illness, the combination of symptoms is unusual. Although he is being treated with a variety of medications, it is unclear whether any of them are helping him or what exactly they are helping him with. (Ironically, once he is off of his medications, he becomes a better dresser and a better dancer.) He writes in a disorganized way in his journal; the only intelligible sentence that is focused on is, “The worst part about having mental illness is people expect you to behave as if you DONT.” A smiley face in the ‘O’ suggests that his affect is inappropriate even in his writing. Arthur’s condition of uncontrollable laughing and/or crying, associated with head trauma, appears more consistent with the neurologic condition pseudobulbar affect rather than a mental illness. In addition to pseudobulbar affect, Arthur demonstrates a constellation of symptoms of different kinds of mental illness, including erotomanic delusions, ideas of reference, and disorganized thinking. He also does not appear to take social cues, such as knowing when he is being mocked. He appears to believe that his neighbor is his girlfriend (as the viewer was similarly led to believe), eventually breaking into her apartment where he thought he belonged, much to her horror when she finds him there. Some of his symptoms may run in his family (whether it be his biological or adoptive family).

Penny (Arthur’s mother) strongly believes (perhaps a delusion, perhaps not) that her previous employer Thomas Wayne (the future Batman’s father) is the father of her love-child, Arthur. When Arthur obtains Penny’s mental health records (through his own violent devices), he finds that she had been diagnosed with narcissistic personality disorder and a psychotic disorder. She had been found guilty of endangering the welfare of her (perhaps adopted, perhaps not) child Arthur, who had been malnourished, with severe head trauma, and tied to a radiator.
Arthur’s smothering of his mother with a pillow in her hospital bed, after he was devastated by both her stroke and this newfound data, occurred in a perfect storm. The killing is not portrayed as an act of euthanasia. We know that schizophrenia is overrepresented among matricide perpetrators and that long-term dysfunctional relationships between mother and (grown) child usually precede matricides. Mothers are often seen as controlling, fathers are often absent (as in Arthur’s case), and the child is often overly dependent. The mother and child (as seen here) often have a relationship marked by love and hate – mutual dependence and hostility. But Arthur is not the only character in the Batman universe to commit matricide. Recall that the Batman’s psychiatrist Amadeus Arkham himself killed his own mentally ill mother during his young adulthood.
Pop culture can give the public negative impressions of mental illness. While filmmakers need not portray actual mental illnesses or their symptoms in moving their stories forward, their portrayals have an impact on what the public sees as mental illness. This is similar to the current American president and others in political power asserting that mental illness causes mass shootings, and those in the public taking their word for it rather than the word of psychiatry.
In actuality, what felt the most true to life in the film was the early scene in which Arthur was seriously assaulted while waving the going-out-of-business sign on the sidewalk, just trying to make a living. As psychiatrists know, people with mental illness are more likely to be victimized by others in society than to be perpetrators of violence. To be sure, some of Arthur’s characteristics are dynamic risk factors, such as his unemployment and social isolation. However, society often conflates mental illness with dangerousness, but most people with mental illness are not violent.
In the final scenes, Arthur Fleck (who is now the Joker) is apparently back in the white-walled Arkham State Hospital, with an implication that he has gotten away with the murders, either found incompetent or insane. This, too, has negative implications for the public viewing the film – and further perpetuates the misunderstanding that people with mental illness “get away” with their crimes. In reality, depending on the study, approximately one-quarter of those who pleaded insanity were found insane, and those facing jury trials (and public perception) are less likely to be found insane than those with bench trials. Public misinterpretations and outrage over the idea that a mentally unwell person might be found insane rather than guilty have existed for centuries, perhaps most memorably when John Hinckley Jr. attempted to assassinate former President Ronald Reagan, after identifying with a character in the film “Taxi Driver.” Let’s presume that Gotham has an insanity defense similar to other places in America. Then, in order to be found insane, Arthur’s pseudobulbar affect or his (unclear) mental illness would have either caused him not to know the nature and consequences of his acts, and/or to appreciate the wrongfulness of his acts (if we are fairly certain that Gotham is actually New York City). Neither of these appear to be true from the film. He knew that he was killing. No delusions or hallucinations made him think his acts were not wrong. Rather, he had an arguably rational motive – certainly the multitudes wearing clown masks in the subsequent uprisings against the powerful also believed his motive to be rational. He deliberately killed the bankers who mocked and beat him. He was also able to defer his killings until what he calculated was the right time to have the most impact – for example, on live television, or when he was alone with his mother in the hospital.
In closing, unrealistic portrayals of the link between mental illness, violence, and forensic hospitalization are seen on the silver screen in “Joker.” We hope that others who feign mental illness symptoms to evade criminal responsibility will emulate Joaquin Phoenix’s Joker as it will make it much easier for forensic psychiatrists to ferret out malingerers!
Dr. Hatters Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington, D.C.: American Psychiatric Association Publishing [2019]), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law. Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and on the faculty at Weill-Cornell Medical Center.
The Batman characters have been cultural icons for generations – spanning more than three-quarters of a century. How many of us had Batman (or the Joker) on our school lunch box or watched reruns of Adam West’s campy televised rendition of Batman? The October release of “Joker” has been breaking contemporary box office records.
(Spoiler alert!) The Todd Phillips film associates mental illness with violent acts, spurring a slew of articles explaining that this association is uncommon and may promote stigmatization and public fear of people with obvious symptoms of mental illness. The protagonist, Arthur Fleck (Joaquin Phoenix), suffers from a condition in which his affect and facial expressions are not appropriate to his emotions or to the situation. He laughs uncontrollably when a situation is sad or upsetting. Sometimes he laughs and cries at the same time. As a result, he often is misunderstood, ridiculed, and victimized – like many people with obvious mental illness.
Arthur Fleck is a loner who has difficulty with relationships and self-esteem, and is beaten severely while at work as a clown. Shortly after the incident, he is given a gun by one of his coworkers. He keeps it with him even when working as a clown in a children’s hospital – where it is accidentally revealed, and he is subsequently fired. Still in his clown garb, he later uses the gun when he is mocked and assaulted on the subway by three Gotham City bankers.
In an unusual tone, his mental health worker reminds him early in the film that he is prescribed seven different psychotropic medications, helping to cement for the viewer that mental illness is the cause of Arthur’s problems and the Joker’s origin story. Then the funding for Arthur’s mental health treatment (even if it was not good treatment) was cut – a problem not just in Gotham.
While some of Arthur Fleck’s symptoms are consistent with real mental illness, the combination of symptoms is unusual. Although he is being treated with a variety of medications, it is unclear whether any of them are helping him or what exactly they are helping him with. (Ironically, once he is off of his medications, he becomes a better dresser and a better dancer.) He writes in a disorganized way in his journal; the only intelligible sentence that is focused on is, “The worst part about having mental illness is people expect you to behave as if you DONT.” A smiley face in the ‘O’ suggests that his affect is inappropriate even in his writing. Arthur’s condition of uncontrollable laughing and/or crying, associated with head trauma, appears more consistent with the neurologic condition pseudobulbar affect rather than a mental illness. In addition to pseudobulbar affect, Arthur demonstrates a constellation of symptoms of different kinds of mental illness, including erotomanic delusions, ideas of reference, and disorganized thinking. He also does not appear to take social cues, such as knowing when he is being mocked. He appears to believe that his neighbor is his girlfriend (as the viewer was similarly led to believe), eventually breaking into her apartment where he thought he belonged, much to her horror when she finds him there. Some of his symptoms may run in his family (whether it be his biological or adoptive family).

Penny (Arthur’s mother) strongly believes (perhaps a delusion, perhaps not) that her previous employer Thomas Wayne (the future Batman’s father) is the father of her love-child, Arthur. When Arthur obtains Penny’s mental health records (through his own violent devices), he finds that she had been diagnosed with narcissistic personality disorder and a psychotic disorder. She had been found guilty of endangering the welfare of her (perhaps adopted, perhaps not) child Arthur, who had been malnourished, with severe head trauma, and tied to a radiator.
Arthur’s smothering of his mother with a pillow in her hospital bed, after he was devastated by both her stroke and this newfound data, occurred in a perfect storm. The killing is not portrayed as an act of euthanasia. We know that schizophrenia is overrepresented among matricide perpetrators and that long-term dysfunctional relationships between mother and (grown) child usually precede matricides. Mothers are often seen as controlling, fathers are often absent (as in Arthur’s case), and the child is often overly dependent. The mother and child (as seen here) often have a relationship marked by love and hate – mutual dependence and hostility. But Arthur is not the only character in the Batman universe to commit matricide. Recall that the Batman’s psychiatrist Amadeus Arkham himself killed his own mentally ill mother during his young adulthood.
Pop culture can give the public negative impressions of mental illness. While filmmakers need not portray actual mental illnesses or their symptoms in moving their stories forward, their portrayals have an impact on what the public sees as mental illness. This is similar to the current American president and others in political power asserting that mental illness causes mass shootings, and those in the public taking their word for it rather than the word of psychiatry.
In actuality, what felt the most true to life in the film was the early scene in which Arthur was seriously assaulted while waving the going-out-of-business sign on the sidewalk, just trying to make a living. As psychiatrists know, people with mental illness are more likely to be victimized by others in society than to be perpetrators of violence. To be sure, some of Arthur’s characteristics are dynamic risk factors, such as his unemployment and social isolation. However, society often conflates mental illness with dangerousness, but most people with mental illness are not violent.
In the final scenes, Arthur Fleck (who is now the Joker) is apparently back in the white-walled Arkham State Hospital, with an implication that he has gotten away with the murders, either found incompetent or insane. This, too, has negative implications for the public viewing the film – and further perpetuates the misunderstanding that people with mental illness “get away” with their crimes. In reality, depending on the study, approximately one-quarter of those who pleaded insanity were found insane, and those facing jury trials (and public perception) are less likely to be found insane than those with bench trials. Public misinterpretations and outrage over the idea that a mentally unwell person might be found insane rather than guilty have existed for centuries, perhaps most memorably when John Hinckley Jr. attempted to assassinate former President Ronald Reagan, after identifying with a character in the film “Taxi Driver.” Let’s presume that Gotham has an insanity defense similar to other places in America. Then, in order to be found insane, Arthur’s pseudobulbar affect or his (unclear) mental illness would have either caused him not to know the nature and consequences of his acts, and/or to appreciate the wrongfulness of his acts (if we are fairly certain that Gotham is actually New York City). Neither of these appear to be true from the film. He knew that he was killing. No delusions or hallucinations made him think his acts were not wrong. Rather, he had an arguably rational motive – certainly the multitudes wearing clown masks in the subsequent uprisings against the powerful also believed his motive to be rational. He deliberately killed the bankers who mocked and beat him. He was also able to defer his killings until what he calculated was the right time to have the most impact – for example, on live television, or when he was alone with his mother in the hospital.
In closing, unrealistic portrayals of the link between mental illness, violence, and forensic hospitalization are seen on the silver screen in “Joker.” We hope that others who feign mental illness symptoms to evade criminal responsibility will emulate Joaquin Phoenix’s Joker as it will make it much easier for forensic psychiatrists to ferret out malingerers!
Dr. Hatters Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington, D.C.: American Psychiatric Association Publishing [2019]), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law. Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and on the faculty at Weill-Cornell Medical Center.
The Batman characters have been cultural icons for generations – spanning more than three-quarters of a century. How many of us had Batman (or the Joker) on our school lunch box or watched reruns of Adam West’s campy televised rendition of Batman? The October release of “Joker” has been breaking contemporary box office records.
(Spoiler alert!) The Todd Phillips film associates mental illness with violent acts, spurring a slew of articles explaining that this association is uncommon and may promote stigmatization and public fear of people with obvious symptoms of mental illness. The protagonist, Arthur Fleck (Joaquin Phoenix), suffers from a condition in which his affect and facial expressions are not appropriate to his emotions or to the situation. He laughs uncontrollably when a situation is sad or upsetting. Sometimes he laughs and cries at the same time. As a result, he often is misunderstood, ridiculed, and victimized – like many people with obvious mental illness.
Arthur Fleck is a loner who has difficulty with relationships and self-esteem, and is beaten severely while at work as a clown. Shortly after the incident, he is given a gun by one of his coworkers. He keeps it with him even when working as a clown in a children’s hospital – where it is accidentally revealed, and he is subsequently fired. Still in his clown garb, he later uses the gun when he is mocked and assaulted on the subway by three Gotham City bankers.
In an unusual tone, his mental health worker reminds him early in the film that he is prescribed seven different psychotropic medications, helping to cement for the viewer that mental illness is the cause of Arthur’s problems and the Joker’s origin story. Then the funding for Arthur’s mental health treatment (even if it was not good treatment) was cut – a problem not just in Gotham.
While some of Arthur Fleck’s symptoms are consistent with real mental illness, the combination of symptoms is unusual. Although he is being treated with a variety of medications, it is unclear whether any of them are helping him or what exactly they are helping him with. (Ironically, once he is off of his medications, he becomes a better dresser and a better dancer.) He writes in a disorganized way in his journal; the only intelligible sentence that is focused on is, “The worst part about having mental illness is people expect you to behave as if you DONT.” A smiley face in the ‘O’ suggests that his affect is inappropriate even in his writing. Arthur’s condition of uncontrollable laughing and/or crying, associated with head trauma, appears more consistent with the neurologic condition pseudobulbar affect rather than a mental illness. In addition to pseudobulbar affect, Arthur demonstrates a constellation of symptoms of different kinds of mental illness, including erotomanic delusions, ideas of reference, and disorganized thinking. He also does not appear to take social cues, such as knowing when he is being mocked. He appears to believe that his neighbor is his girlfriend (as the viewer was similarly led to believe), eventually breaking into her apartment where he thought he belonged, much to her horror when she finds him there. Some of his symptoms may run in his family (whether it be his biological or adoptive family).

Penny (Arthur’s mother) strongly believes (perhaps a delusion, perhaps not) that her previous employer Thomas Wayne (the future Batman’s father) is the father of her love-child, Arthur. When Arthur obtains Penny’s mental health records (through his own violent devices), he finds that she had been diagnosed with narcissistic personality disorder and a psychotic disorder. She had been found guilty of endangering the welfare of her (perhaps adopted, perhaps not) child Arthur, who had been malnourished, with severe head trauma, and tied to a radiator.
Arthur’s smothering of his mother with a pillow in her hospital bed, after he was devastated by both her stroke and this newfound data, occurred in a perfect storm. The killing is not portrayed as an act of euthanasia. We know that schizophrenia is overrepresented among matricide perpetrators and that long-term dysfunctional relationships between mother and (grown) child usually precede matricides. Mothers are often seen as controlling, fathers are often absent (as in Arthur’s case), and the child is often overly dependent. The mother and child (as seen here) often have a relationship marked by love and hate – mutual dependence and hostility. But Arthur is not the only character in the Batman universe to commit matricide. Recall that the Batman’s psychiatrist Amadeus Arkham himself killed his own mentally ill mother during his young adulthood.
Pop culture can give the public negative impressions of mental illness. While filmmakers need not portray actual mental illnesses or their symptoms in moving their stories forward, their portrayals have an impact on what the public sees as mental illness. This is similar to the current American president and others in political power asserting that mental illness causes mass shootings, and those in the public taking their word for it rather than the word of psychiatry.
In actuality, what felt the most true to life in the film was the early scene in which Arthur was seriously assaulted while waving the going-out-of-business sign on the sidewalk, just trying to make a living. As psychiatrists know, people with mental illness are more likely to be victimized by others in society than to be perpetrators of violence. To be sure, some of Arthur’s characteristics are dynamic risk factors, such as his unemployment and social isolation. However, society often conflates mental illness with dangerousness, but most people with mental illness are not violent.
In the final scenes, Arthur Fleck (who is now the Joker) is apparently back in the white-walled Arkham State Hospital, with an implication that he has gotten away with the murders, either found incompetent or insane. This, too, has negative implications for the public viewing the film – and further perpetuates the misunderstanding that people with mental illness “get away” with their crimes. In reality, depending on the study, approximately one-quarter of those who pleaded insanity were found insane, and those facing jury trials (and public perception) are less likely to be found insane than those with bench trials. Public misinterpretations and outrage over the idea that a mentally unwell person might be found insane rather than guilty have existed for centuries, perhaps most memorably when John Hinckley Jr. attempted to assassinate former President Ronald Reagan, after identifying with a character in the film “Taxi Driver.” Let’s presume that Gotham has an insanity defense similar to other places in America. Then, in order to be found insane, Arthur’s pseudobulbar affect or his (unclear) mental illness would have either caused him not to know the nature and consequences of his acts, and/or to appreciate the wrongfulness of his acts (if we are fairly certain that Gotham is actually New York City). Neither of these appear to be true from the film. He knew that he was killing. No delusions or hallucinations made him think his acts were not wrong. Rather, he had an arguably rational motive – certainly the multitudes wearing clown masks in the subsequent uprisings against the powerful also believed his motive to be rational. He deliberately killed the bankers who mocked and beat him. He was also able to defer his killings until what he calculated was the right time to have the most impact – for example, on live television, or when he was alone with his mother in the hospital.
In closing, unrealistic portrayals of the link between mental illness, violence, and forensic hospitalization are seen on the silver screen in “Joker.” We hope that others who feign mental illness symptoms to evade criminal responsibility will emulate Joaquin Phoenix’s Joker as it will make it much easier for forensic psychiatrists to ferret out malingerers!
Dr. Hatters Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington, D.C.: American Psychiatric Association Publishing [2019]), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law. Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and on the faculty at Weill-Cornell Medical Center.
Postpartum psychosis: Protecting mother and infant
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
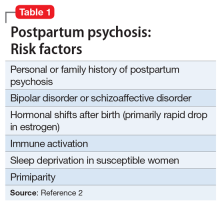
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
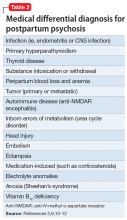
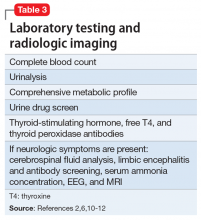
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
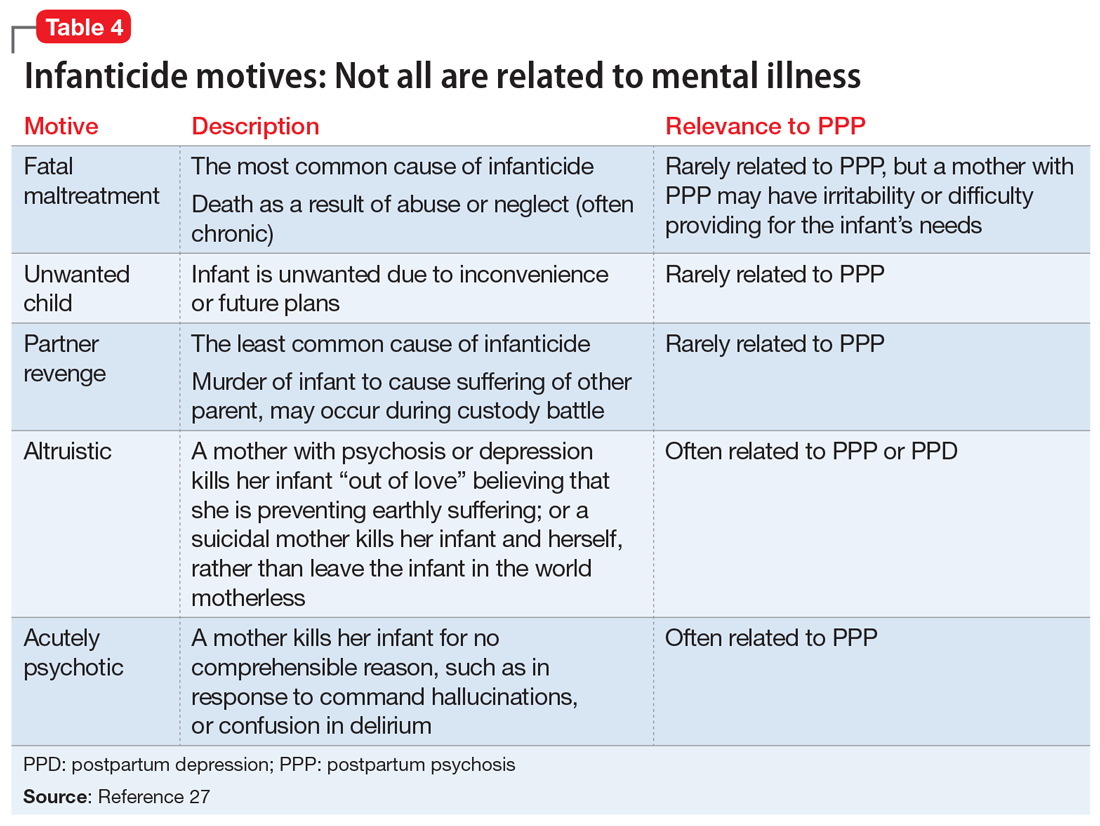
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
Psychiatric considerations in menopause
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
Sexting: What are the clinical and legal implications?
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
Antidepressant use during pregnancy: How to avoid clinical and legal pitfalls
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
Does your OB patient have a psychiatric complaint? And can you manage it?
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
There’s a full moon tonight—and you’re the obstetrician on call. Not that you should expect any more funny business than usual. Despite stories of werewolves and other deviants coming out of the woodwork, there is no “full moon effect”—at least not one that can be documented. Nevertheless, chances are good that you will encounter at least one of the following psychiatric challenges as you end your day in the clinic and move on to an extended vigil:
- postpartum depression
- leaving against medical advice
- agitation
- antenatal illicit drug use
- denial or concealment of pregnancy.
In this article, we describe the management of these challenges and make recommendations to help increase your comfort level with patients who exhibit psychiatric problems. In some situations, our suggestions may help you manage the problem without a psychiatric consult.
Postpartum depression
CASE 1: Is it just the blues?
It is the end of your day in the clinic, and your last patient is a 30-year-old G3P3 who is 6 weeks postpartum. She describes repeated tearful episodes over the course of several weeks, decreased concentration, and poor appetite. She feels guilty because she is tired all the time and not bonding with her baby. She denies having suicidal or homicidal thoughts, or any hallucinations. She had expected her energy to return to normal over the first few postpartum weeks, but it has not. She is worried because she will soon be returning to work as a medical resident.
Does this patient have postpartum depression? Or is it another condition with overlapping symptoms?
If a mother tells you that she is suicidal or having thoughts of harming her child or others, she should be sent immediately to the nearest emergency department for psychiatric evaluation. Short of such a dramatic situation, how do you know when you should manage a patient’s depression on your own and when she should see a psychiatrist? Thorough assessment is the key.
Don’t mistake transient feelings for depression
Transient feelings of sadness, bereavement, and grief are not the same as depression, which must last 2 weeks or longer to confirm the diagnosis.
A quick mnemonic for symptoms of depression is SIG: E CAPS (as if writing a prescription for energy capsules) (TABLE 1).1 This mnemonic helps remind you to assess the patient’s sleep, interest, guilt, energy, concentration, appetite, and psychomotor function, as well as identify any suicidal ideation.
It is important to assess a woman’s sleep and appetite in addition to mood. However, differences may be difficult to ascertain due to normal changes in the postpartum period. One useful question is whether the mother is able to sleep when the baby sleeps. If she isn’t, this wakefulness may be a symptom of depression.
The Edinburgh Postnatal Depression Scale is an easy, 10-question screening tool that is completed by the patient; it can be used both during pregnancy and postpartum. It is available on the Web at a number of sites, including www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf.
TABLE 1
SIG: E CAPS—a mnemonic to assess for depression1
| Decreased (sometimes increased) Sleep |
| Decreased Interests |
| Feelings of Guilt |
| Decreased Energy |
| Decreased Concentration |
| Decreased (sometimes increased) Appetite |
| Psychomotor retardation, slowness |
| Suicidal thoughts, plans, or intent |
Differential diagnosis
Besides postpartum depression, the differential diagnosis for altered mood in the postpartum period includes several entities.
Baby blues generally occurs quite soon after birth and resolves within 2 weeks. It involves crying, emotional lability, and irritability.2 It occurs in around 50% to 75% of new mothers (compared with postpartum depression, which affects 10% to 20%).3-5
Postpartum psychosis often involves the onset of psychotic symptoms within 1 week after delivery. The patient may exhibit both mood symptoms and psychosis. For example, she may believe that the baby is not hers or hear voices commanding her to kill the baby or warning her not to trust her healthcare providers.6 Postpartum psychosis has a prevalence of about 0.2%.3-6
This psychosis can be organic in nature or can arise from a preexisting mood disorder or schizophrenia. Because treatment varies, depending on the cause, a thorough medical workup is needed.
Bipolar disorder may present as depression, but it also consists of manic periods of elevated, expansive, or irritable mood that last several days to weeks. Many symptoms appear to be the opposite of depression, such as increased energy and elevated self-esteem.7,8
It is important to consider bipolar disorder in the differential diagnosis. If a woman who has unrecognized bipolar disorder is given an antidepressant, a manic state could be precipitated. Women who have bipolar disorder require different drugs than women who have depression only, and they should be evaluated by a psychiatrist, at least initially.
Start treatment as soon as possible
Once you confirm that the patient has postpartum depression—and not another psychiatric disorder—prescribing an antidepressant may be the next step. Keep in mind that these drugs take several weeks before their benefits are felt. Therefore, it is best to start an antidepressant before depression becomes severe. The mother may also benefit from psychotherapy.
The selective serotonin reuptake inhibitor sertraline (Zoloft) is a reasonable first choice in pregnancy and lactation when the depression is of new onset.9,10 Start it gradually (e.g., 25 mg for sertraline, which can cause nausea if it is initiated too rapidly) and titrate it over time, if necessary. When there is comorbid anxiety, it sometimes is helpful to prescribe low dosages of lorazepam (Ativan, Temesta) on an as-needed basis, while the patient is waiting for the antidepressant to “kick in.” Also consider follow-up—do you plan to follow her frequently or refer her to psychiatry?
Adjunctive or alternative options include psychotherapy, group therapy, and music therapy. Referral to a psychiatrist is warranted if the patient does not respond to the initial antidepressant agent.
Also be aware that untreated depression can become so severe that a woman can begin to experience psychosis, warranting rapid referral. Also refer any woman who reports a complex history of previous depression—unless the previous episode was easily controlled with a medicine safe for use during pregnancy and lactation.
If the patient is not lactating, a greater range of agents may be considered. (A full discussion of the risks and benefits of antidepressant use in pregnancy is outside the scope of this article. The interested reader is referred to an article on the subject by Wisner and colleagues.11)
CASE 1 RESOLVED
A comprehensive discussion with the mother reveals that she is suffering from postpartum depression. No history of bipolar or psychotic symptoms is discovered. After discussing treatment options, you prescribe sertraline. Over the next 2 months, the patient’s symptoms improve, and she bonds with her infant and successfully returns to work. She is also referred to a psychologist to work through some underlying issues.
Leaving against medical advice
CASE 2: Patient threatens to leave the hospital
At midnight, you are paged to attend to a 32-year-old G1P0 at 27 weeks’ gestation who is threatening to leave against medical advice. She was admitted earlier in the day with uncontrolled gestational diabetes and is refusing her insulin.
How do you respond?
Use the relationship that you have established with this patient to the best of your ability. Make sure that you have explained fully, and in language she can easily comprehend, the reasons she needs to stay for treatment.
Don’t overlook the obvious, either: Why does she want to leave? Sometimes the reason makes sense (e.g., one mother wanted to leave to protect her daughter from an abusive husband). Other reasons may be related to psychosis, addiction, lack of sleep in the hospital, or a desire to smoke, drink, or use drugs. Can you convince her to postpone her decision until morning, when her physician will be available?
It is important to document in the medical record your explanations and her reasoning. Can she coherently verbalize an understanding of the consequences of her decision to leave, including the risks and implications to herself and the fetus?12 Can she describe alternatives and the reasoning against them?
If she is able to do these things, and you find her thought processing and reasoning to be lucid, then she may have the capacity to leave against medical advice. Keep in mind that rational persons do have the right, constitutionally, to refuse treatment, even if doing so will lead to morbidity. (A Jehovah’s Witness who refuses treatment is the typical example.12) Contact the hospital’s attorney—tonight—and document that you did so. The attorney may recommend that the patient sign a letter stating that she recognizes the maternal and fetal risks of leaving.
Sometimes a patient must be held against her will
Some mothers lack the capacity to refuse treatment. They may be unable to verbalize an understanding of the situation and its risks. Their reasoning may be abnormal, with disorganized or delusional thinking, or both. The patient may be tangential or talk “in circles” rather than answer your questions.
Try to ascertain whether mood symptoms are contributing to her irrational thinking. For example, is her rationale for going home—“just to be with my husband because I don’t want to be alone”—due to her depression, despite the risk to herself and the fetus? Try to be flexible and creative. For example, you could call the husband and ask him to come to the hospital to sit with the patient.
Is the patient psychotic? For example, does she believe she has to leave now because the staff has been replaced by aliens who plan to kill her and her fetus? If so, you have the authority to continue her hospitalization—but contact the psychiatry department for medication recommendations. A urine toxicology screen would also be prudent.
If the patient is irrational and lacks the capacity to decide whether to stay or leave, document your conversation with her, as well as the reasoning behind your decision to intervene further. Other steps include:
- contacting the hospital’s attorney
- completing an emergency detention form
- calling security
- ensuring that the patient’s environment is safe for her and others (TABLE 2).13
TABLE 2
5 steps to sound management of a patient who wants to leave
against medical advice
| 1. Ask the patient why she wants to leave now |
| 2. Inform her of the risks to herself and to her fetus |
| 3. Ask her to verbalize the risks to herself and to her fetus |
4. Determine whether the patient’s request is rational
|
| 5. Document the medical explanation and reasoning in the chart |
CASE 2 RESOLVED
After building some rapport with the patient, you ask why she wants to leave right now. During this conversation, the patient reveals that she has not slept in three nights, and says she believes that the insulin is keeping her up. You are able to assure her that this is not the case and offer her something to help her sleep. She decides not to leave against medical advice.
Unexplained agitation
CASE 3: Patient becomes abusive
At 1 AM, you are called to the seventh floor, where a 20-year-old G2P1 at 26 weeks’ gestation is yelling at staff and hitting anyone who comes near. She was admitted earlier in the day for management of threatened abortion and a dilated cervix. She has no documented psychiatric history, but is flushed, disheveled, and hostile, accusing the staff of sabotaging her life, and is seen picking at imaginary things. You notify psychiatry, but no one is available.
What do you do?
Determining the origin of these symptoms will help determine the appropriate course of action. Among the possibilities are:
- drug intoxication or withdrawal
- delirium
- psychosis
- a chronic problem such as a personality disorder (TABLE 3).
Psychosis means that a patient is out of touch with reality. A psychotic patient may experience delusions, auditory and visual hallucinations, and gross disorganization. Brief psychotic episodes usually last for 1 day to 1 month, with eventual recovery to premorbid functioning.7
Substances such as medications or illicit drugs also can induce psychosis. Major offenders include steroids and narcotic agents. Alternatively, sudden withdrawal of illicit substances (due to hospitalization) could manifest as delirium or psychosis.
Personality disorder. If the patient’s behavior is not new but a long-term problem, she may have a chronic personality disorder rather than acute illness. Personality problems involve pervasive response patterns and dysfunctional coping patterns that affect daily life. For example, a patient who has borderline personality disorder may have emotional instability presenting as intense episodic dysphoria or irritability. Such patients have a hard time empathizing with others, poor impulse control, and a desire for instant gratification. They may also misinterpret the behavior of other people and take offense easily as a result. Lacking stress-management skills, they regress to unhealthy defense mechanisms such as acting out, complaining, passive aggressiveness, and splitting of the staff (thinking that people are all good or all bad).
Dementia may also be on the differential diagnosis, but this chronic condition is unlikely in such a young patient (unless she were in the late stages of HIV/AIDS, for example). Dementia has a gradual onset and is irreversible.
TABLE 3
Some causes of agitation
Delirium
|
Psychosis
|
Dementia
|
Personality dysfunction
|
Workup for the agitated patient
Assess vital signs and basic laboratory studies, particularly a complete blood count, thyroid testing, metabolic screens, glucose, serum chemistry panel, and urine toxicology, to rule out causes of delirium and detect any substances the patient may have used. Also consider that the patient may have initiated a new medication recently.
Imaging of the brain or chest or electroencephalography may be necessary, such as in the setting of infection or concerns regarding seizure activity.
Gather collateral information about the patient from her relatives, friends, and the support staff. Search her chart for recent contacts. Did she have a visitor or phone call that may have upset her? Explore whether she is having problems with her partner or family and friends. Also confirm that her agitated behavior is an acute change.
Investigate the patient’s paranoia. Why does she believe that the staff is against her? Does she believe they are trying to harm, kill, or poison her? Assess her reasoning to determine whether her behavior is psychotic or a personality problem.
Ask her about hallucinations, keeping in mind that hallucinations are different from illusions, in which a patient misinterprets what she sees. What are the voices saying to her?
Also ask about any suicidal or homicidal commands. If she acknowledges that she is hearing them, get a sitter for her immediately and make her environment safe so that she is unable to harm herself. Then contact the psychiatry department again.
How to intervene
Talk gently and quietly in an attempt to calm the situation. Try to make yourself “small”: Stand back and stay at the patient’s eye level, not in her personal space or towering over her.
Also, protect yourself. Don’t challenge her complaints immediately or you will alienate her. Medically evaluate and treat the cause of her agitation, and, if medications are necessary for psychosis or sedation, contact psychiatry for assistance.
CASE 3 RESOLVED
The patient does not respond to your attempts to reason and refuses to allow the nurses to check her vital signs. Security is called to stand by while her vital signs are reassessed. The nurses inform you that the patient’s family is in the waiting room. Though you find no documented history of substance use or abnormal labs, the family reports that the patient had a history of alcohol abuse but quit drinking about 3 days earlier. They also report that, before she quit, she drank approximately 25 oz of vodka and a sixpack of beer nightly. They deny knowledge of any other illicit drug use. Because her vital signs suggest alcohol withdrawal, you offer oral lorazepam and treat her according to the hospital’s alcohol withdrawal protocol. She recovers without any further complications and is referred to the chemical dependency service for evaluation.
Drug abuse during pregnancy
CASE 4: Patient skips prenatal care
It is 2 AM, and you are about to get some rest when you are paged by the emergency room about a 26-year-old G5P4, who is in active labor with no dates. You check the database and discover that her previous pregnancies were complicated by chronic substance abuse.
How do you respond?
You might feel frustrated and angry with this patient. Considering that you have not met her, these feelings would be based on previous contacts with patients who had a similar history. This is countertransference. We all experience it. It can be helpful or harmful, but you cannot control it unless you are aware of it. Pay attention to the anger, happiness, or pride that a patient triggers in you, and acknowledge that it is your issue.
Your frustration may also stem from personal feelings about mothers who repeatedly expose their fetuses to drugs and neglect prenatal care, as well as anxiety about what you are legally and ethically bound to do.
In Ferguson v City of Charleston, the Supreme Court found that drug testing of a pregnant woman for the purpose of criminal prosecution is a violation of Fourth Amendment rights.14 However, there have been cases in which a state prosecuted a woman for using an illegal substance during pregnancy.
These cases involved:
- child neglect15
- delivery of a stillborn fetus whose autopsy revealed traces of cocaine by-products16
- reckless endangerment after a newborn tested positive for cocaine.17
What is drug abuse?
According to the 4th edition of the Diagnostic and Statistical Manual of Mental Health Disorders, it is a “maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances” within a 12-month period, or persistently.7 To meet criteria for substance dependence, in addition to the criteria just mentioned, the individual must be tolerant to the drug, experience withdrawal when the drug is cut back or stopped, continue to use the drug despite knowledge of its dangers, or all of the above. Mothers who match this description often lose custody of their child—sometimes to foster care, sometimes to other family members.20,21
Some states use positive serum and urine toxicology as evidence to remove a child from the mother’s custody.19
It is important to attempt to build a doctor–patient relationship. You cannot solve the patient’s substance dependence problem in one night, but you can refer her to drug treatment. A urine toxicology screen can help you and the pediatricians know what the patient has been exposed to (TABLE 4).
TABLE 4
Commonly abused drugs and their potential effects
| Drug | Withdrawal effects on mother | Drug effects on fetus or infant |
|---|---|---|
| Alcohol | Sweating, increased heart rate, hand tremor, nausea/vomiting, physical agitation, hallucination (tactile, visual, auditory), illusions, grand mal seizures25 | Fetal alcohol syndrome. Withdrawal symptoms similar to those of the mother |
| Cocaine | Agitation, anxiety, anger, nausea/vomiting, muscle pain, disturbed sleep, depression, intense cravings for the drug, irritability25 | Risk of abruptio placenta, small-for-gestational-age infant, microcephaly, congenital anomaly (cardiac and genitourinary abnormality, necrotizing enterocolitis), central nervous system stroke or hemorrhage. Withdrawal effects include hypertonia, jitteriness, and seizures.26 |
| Crystal methamphetamine | Anxiety, psychotic reaction, intense hunger, irritability, restlessness, fatigue, depression, sleep disturbance, cravings25 | Premature birth, abruptio placenta, small-for-gestational age, hypertonia, tremors, poor feeding, abnormal sleep patterns26 |
| Marijuana | Irritability, anxiety, physical tension, decreased appetite and mood25 | Irritability, increase in bodily motility, tremors, startles, poor habituation to visual stimuli, abnormal reflexes, symptoms similar to mild withdrawal27 |
| Opioids (Heroin, methadone) | Dilated pupils, watery eyes, runny nose, diarrhea, nausea/vomiting, muscle cramps, piloerection, chills or profuse sweating, yawning, loss of appetite, tremor, jitteriness, panic, insomnia, stomach ache, irritability26 | Risk of prematurity, small-for-gestational age, adult withdrawal symptoms, irritability, hypertonia, wakefulness, jitteriness, diarrhea, increased hiccups, yawning and sneezing, excessive sucking and seizures. Withdrawal effects occur earlier in heroin-exposed babies than in methadone-exposed infants.26 |
CASE 4 RESOLVED
After delivery, a test indicates that the newborn has been exposed to cocaine. The mother admits to cocaine use during pregnancy. She says she did not seek prenatal care because she was afraid of being prosecuted and sent to jail. A social work consult is requested, and the mother is referred to a substance abuse treatment program. State law requires the case to be reported to child protective services. Upon hospital discharge, the newborn is initially placed with the paternal grandmother.
Denial of pregnancy
CASE 5: Patient’s labor takes her by surprise
The night is nearing its end, but it isn’t over yet. At 5:30 AM, you are called to a precipitous delivery involving a 17-year-old who has had no prenatal care. She denies knowing that she was pregnant, and says she thought her labor pains were a bowel movement. Her parents were similarly unaware that their daughter was pregnant, and are threatening to disown her.
How do you defuse the situation?
In a study of women who denied or concealed pregnancy, patients presented to the hospital for various reasons.22 For example, one woman went to the ER because she was seizing and her workup revealed that she had eclampsia. A number of women did not even recognize when they were in labor. The infants born to these women are at risk for a poor neonatal outcome.21,22
How can psychiatry help in such a case? By determining whether the patient denied her pregnancy—even to herself—or actively concealed it from others. Obviously, these circumstances have differing implications.
Denial is not a simple entity. It may involve a psychotic schizophrenic woman who is out of touch with the reality of her pregnancy; a woman who “affectively” denies her pregnancy, keeping the significance of her condition from herself and behaving as though she is not gravid (perhaps because she plans to give the baby up for adoption); or a woman who has pervasive denial and does not know that she is pregnant.22,23 In contrast, a woman who conceals her pregnancy is quite aware that she is gravid but consciously hides the gestation from others, begging the question of what she had planned for the future.22
Psychological issues abound, and may include a history of sexual and psychological trauma, an attempt to avoid religious prohibitions against unwed intercourse, anger at the father of the infant, and even homicidal urges toward the baby.24 There may be more going on under the surface than “only” a failure to recognize the pregnancy, and the patient may need further mental health treatment.
Consider how well this young woman can be a mother. When she did not even recognize that she was pregnant for 9 months, how well will she be able to attend to her baby’s needs? Psychiatry can evaluate the patient to help determine her capacity for parenting and whether child protective services should be alerted. Of additional concern is the distress of the patient’s parents. Family support will be extremely important.
Be sure to conduct thorough contraceptive education and planning at the time of discharge because this patient is at risk for future denied or concealed pregnancies.22
CASE 5 RESOLVED
The patient is seen by psychiatry. She has no major mental illness, but her denial appears to be related to problems with her boyfriend, her attempts to be the perfect daughter, and fear of being disowned. After the initial shock, the patient’s parents become more supportive and begin to bond with their new grandchild. The new mom is educated about birth control and agrees to follow up with a counselor and take parenting classes. The baby is discharged to his mother and grandparents.
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-
1. What does SIGECAPS stand for? Available at: www.acronymfinder.com/Sleep,-Interest,-Guilt,-Energy,-Concentration,-Appetite,-Psychomotor,-Suicidal-(mnemonic-for-characteristics-of-major-depression)-(SIGECAPS).html. Accessed May 18, 2009.
2. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64:1284-1292.
3. Grigoriadis S, Romans S. Postpartum psychiatric disorders: what do we know and where do we go? Curr Psychiatry Rev. 2006;2:151-158.
4. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23:188-193.
5. Sadock BJ, Sadock VA. Psychiatry and reproductive medicine. In: Kaplan HI, Sadock BJ, eds. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2003:868-878.
6. Friedman SH, Resnick PJ, Rosenthal M. Postpartum psychosis: strategies to protect infant and mother from harm. Curr Psychiatry. 2009;8(2):40-46.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: American Psychiatric; 2000.
8. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
9. Gentile S. Use of contemporary antidepressants during breastfeeding: a proposal for a specific safety index. Drug Saf. 2007;30:107-121.
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22:571-575.
11. Wisner KL, Zarin DA, Holmboe ES, et al. Riskbenefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933.-
12. Roberts LW, Hoop JG. Professionalism and ethics. Washington, DC: American Psychiatric Publishing; 2008.
13. Ohio Criteria for Commitment, Section 5122.01.
14. Harris LH, Paltrow L. The status of pregnant women and fetuses in US criminal law. JAMA. 2003;289:1697-1699.
15. Drugs, Police & The Law: Whitner vs. the State of South Carolina. June 23, 2004. Available at: www.drugpolicy.org/law/womenpregnan/whitnervsth_/. Accessed April 30, 2009.
16. Gray P. Prosecution of prenatal substance abuse allowed to stand in McKnight case. Available at: www.law.uh.edu/healthlaw/perspectives/reproductive/040202prosecution.pdf. Accessed April 30, 2009.
17. Parks AW. New mothers fight endangerment convictions. Public Justice Center. April 10, 2006. Available at: www.publicjustice.org/news/index.cfm?newsid=106. Accessed April 30, 2009.
18. Parks AW. Using cocaine while pregnant is not reckless endangerment. The Daily Record. August 4, 2006. Available at: www.publicjustice.org/pdf/Cruzdailyrecord.pdf. Accessed April 30, 2009.
19. American Pregnancy Association. Using illegal street drugs during pregnancy. May 2007. Available at: www.americanpregnancy.org/pregnancyhealth/illegaldrugs.html. Accessed April 30, 2009.
20. Neuspiel DR, Zingman TM, Templeton VH, et al. Custody of cocaine-exposed newborns: determinants of discharge decisions. Am J Public Health. 1993;83:1726-1729.
21. Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abuse Negl. 2009;33:116-122.
22. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48:117-122.
23. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. Washington, DC: American Psychiatric Publishing; 2003.
24. Bonnet C. Adoption at birth: prevention against abandonment or neonaticide. Child Abuse Negl. 1993;17:501-513.
25. Heroin withdrawal. Available at: www.addictionwithdrawal.com/heroin.htm. Accessed April 29, 2009.
26. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. 1997;43:235-242.
27. Bada HS, Reynolds EW, Hansen WF. Marijuana use, adolescent pregnancy, and alteration in new born behavior: how complex can it get? J Pediatr. 2006;149:742.-
Postpartum psychosis: Strategies to protect infant and mother from harm
In June 2001, Andrea Yates drowned her 5 children ages 6 months to 7 years in the bathtub of their home. She had delusions that her house was bugged and television cameras were monitoring her mothering skills. She came to believe that “the one and only Satan” was within her, and that her children would burn in hell if she did not save their souls while they were still innocent.
Her conviction of capital murder in her first trial was overturned on appeal. She was found not guilty by reason of insanity at her retrial in 2006 and committed to a Texas state mental hospital.1
Postpartum psychosis (PPP) presents dramatically days to weeks after delivery, with wide-ranging symptoms that can include dysphoric mania and delirium. Because untreated PPP has an estimated 4% risk of infanticide (murder of the infant in the first year of life),2 and a 5% risk of suicide,3 psychiatric hospitalization usually is required to protect the mother and her baby.
The diagnosis may be missed, however, because postpartum psychotic symptoms wax and wane and suspiciousness or poor insight cause some women—such as Andrea Yates—to hide their delusional thinking from their families. This article discusses the risk factors, prevention, and treatment of PPP, including a review of:
- infanticide and suicide risks in the postpartum period
- increased susceptibility to PPP in women with bipolar disorder and other psychiatric disorders
- hospitalization for support and safety of the mother and her infant.
Risks of infanticide and suicide
A number of motives exist for infanticide (Table 1).4 Psychiatric literature shows that mothers who kill their children often have experienced psychosis, suicidality, depression, and considerable life stress.5 Common factors include alcohol use, limited social support, and a personal history of abuse. Studies on infanticide found a significant increase in common psychiatric disorders and financial stress among the mothers. Neonaticide (murder of the infant in the first day of life) generally is not related to PPP because PPP usually does not begin until after the day of delivery.6
Among women who develop psychiatric illness, homicidal ideation is more frequent in those with a perinatal onset of psychopathology.7 Infanticidal ideas and behavior are associated with psychotic ideas about the infant.8 Suicide is the cause of up to 20% of postpartum deaths.9
Table 1
Motives for infanticide: Mental illness or something else?
| Motives | Examples |
|---|---|
| Likely related to postpartum psychosis or depression | |
| Altruistic | A depressed or psychotic mother may believe she is sending her baby to heaven to prevent suffering on earth |
| A suicidal mother may kill her infant along with herself rather than leave the child alone | |
| Acutely psychotic | A mother kills her baby for no comprehensible reason, such as in response to command hallucinations or the confusion of delirium |
| Rarely related to postpartum psychosis | |
| Fatal maltreatment | ‘Battered child’ syndrome is the most common cause of infanticide; death often occurs after chronic abuse or neglect |
| A minority of perpetrators are psychotic; a mother out of touch with reality may have difficulty providing for her infant’s needs | |
| Not likely related to postpartum psychosis | |
| Unwanted child | Parent does not want child because of inconvenience or out-of-wedlock birth |
| Spouse revenge | Murder of a child to cause emotional suffering for the other parent is the least frequent motive for infanticide |
| Source: Reference 4 | |
The bipolar connection
Many factors can elevate the risk of PPP, including sleep deprivation in susceptible women, the hormonal shifts after birth, and psychiatric comorbidity (Table 2). Nearly three-fourths (>72%) of mothers with PPP have bipolar disorder or schizoaffective disorder, whereas 12% have schizophrenia.10 Some authors consider PPP to be bipolar disorder until proven otherwise. Mothers with a history of bipolar disorder or PPP have a 100-fold increase in rates of psychiatric hospitalization in the postpartum period.11
Presentation. PPP is relatively rare, occurring at a rate of 1 to 3 cases per 1,000 births. Symptoms often have an abrupt onset, within days to weeks of delivery.10 In at least one-half of cases, symptoms begin by the third postpartum day,13 when many mothers have been discharged home and may be solely responsible for their infants.
Symptoms include confusion, bizarre behaviors, hallucinations (including rarer types such as tactile and olfactory), mood lability (ranging from euphoria to depression), decreased need for sleep or insomnia, restlessness, agitation, disorganized thinking, and bizarre delusions of relatively rapid onset.13 One mother might believe God wants her baby to be sacrificed as the second coming of the Messiah, a second may believe she has special powers, and a third that her baby is defective.
Table 2
Postpartum psychosis: Risk factors supported by evidence
| Sleep deprivation in susceptible women |
| Hormonal shifts after birth (primarily the rapid drop in estrogen) |
| Psychosocial stressors such as marital problems, older age, single motherhood, lower socioeconomic status |
| Bipolar disorder or schizoaffective disorder |
| Past history of postpartum psychosis |
| Family history of postpartum psychosis |
| Previous psychiatric hospitalization, especially during the prenatal period for a bipolar or psychotic condition |
| Menstruation or cessation of lactation |
Obstetric factors that can cause a small increase in relative risk:
|
| Source: For bibliographic citations |
Differential diagnosis
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms (Box).11
Postpartum depression (PPD) occurs in approximately 10% to 15% of new mothers.14 Depressive symptoms occur within weeks to months after delivery and often coexist with anxious symptoms. Some women with severe depression may present with psychotic symptoms. A mother may experience insomnia, sometimes not being able to sleep when the baby is sleeping. She may lack interest in caring for her baby and experience difficulty bonding.
At times it can be difficult to distinguish PPD from PPP. When evaluating a mother who is referred for “postpartum depression,” consider PPP in the differential diagnosis. A woman with PPD or PPP may report depressed mood, but in PPP this symptom usually is related to rapid mood changes. Other clinical features that point toward PPP are abnormal hallucinations (such as olfactory or tactile), hypomanic or mixed mood symptoms, and confusion.
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms, giving special consideration to metabolic, neurologic, cardiovascular, infectious, and substance- or medication-induced origins. The extensive differential diagnosis includes:
- thyroiditis
- tumor
- CNS infection
- head injury
- embolism
- eclampsia
- substance withdrawal
- medication-induced (such as corticosteroids)
- electrolyte anomalies
- anoxia
- vitamin B12 deficiency.11
Psychosis vs OCD. Psychotic thinking and behaviors also must be differentiated from obsessive thoughts and compulsions.10,16 Obsessive compulsive disorder (OCD) may be exacerbated or emerge for the first time during the perinatal period.17
In postpartum OCD, women may experience intrusive thoughts of accidental or purposeful harm to their baby. As opposed to women with PPP, mothers with OCD are not out of touch with reality and their thoughts are ego-dystonic.17 When these mothers have thoughts of their infants being harmed, they realize that these thoughts are not plans but fears and they try to avoid the thoughts.
Preventing PPP
Bipolar disorder is one of the most difficult disorders to treat during pregnancy because the serious risks of untreated illness must be balanced against the potential teratogenic risk of medications. Nevertheless, proactively managing bipolar disorder during pregnancy may reduce the risk of PPP.10
Closely monitor women with a history of bipolar disorder or PPP. During pregnancy, counsel them—and their partners—to:
- anticipate that depressive or psychotic symptoms could develop within days after delivery18
- seek treatment immediately if this occurs.
Postpartum medication. Whether or not a woman with bipolar disorder takes medication during pregnancy, consider treatment with mood stabilizers or atypical antipsychotics in the postpartum to prevent PPP (Table 3). Evidence is limited, but a search of PubMed found 1 study in which prophylactic lithium was given late in the third trimester or immediately after delivery to 21 women with a history of bipolar disorder or PPP. Only 2 patients had a psychotic recurrence while on prophylactic lithium; 1 unexplained stillbirth occurred.19
A retrospective study examined the course of women with bipolar disorder, some of whom were given prophylactic mood stabilizers immediately in the postpartum. One of 14 who received antimanic agents relapsed within the first 3 months postpartum, compared with 8 of 13 who were not so treated.18
Compared with antiepileptics, less information is available about the use of atypical antipsychotics in pregnancy and lactation. Antipsychotics’ potential advantage in women at risk for PPP is that these agents may help prevent or treat both manic and psychotic symptoms.
In a small, naturalistic, prospective study, 11 women at risk for PPP received olanzapine alone or with an antidepressant or mood stabilizer for at least 4 weeks after delivery. Two (18%) experienced a postpartum mood episode, compared with 8 (57%) of 14 other at-risk women who received antidepressants, mood stabilizers, or no medication.20
When you discuss breast-feeding, consider possible risks to the neonate as well as potential sleep interruption for the mother. If a mother has a supportive partner, the partner might be put in charge of night-time feedings in a routine combining breast-feeding and bottle-feeding. In some cases you may need to recommend cessation of lactation.21
Table 3
Treating postpartum psychosis: Consider 3 components
| Component | Recommendations |
|---|---|
| Hospitalization vs home care | Hospitalize in most cases because of emergent severe symptoms and fluctuating course; base decision on risk evaluation/safety issues for patient and infant After discharge, visiting nurses are useful to help monitor the mother’s condition at home |
| Psychoeducation | Educate patient, family, and social support network; address risks to mother and infant and risks in future pregnancies |
| Medication | When prescribing mood stabilizers and/or antipsychotics, consider:
|
Managing PPP
Early symptoms. Because of its severity and rapid evolution, PPP often presents as a psychiatric emergency. Monitor atrisk patients’ sleep patterns and mood for early signs of psychosis.22 Watch especially for hypomanic symptoms such as elevated or mixed mood and decreased judgment, which are common early in PPP.13
A mother with few signs of abnormal mood, good social support, and close follow-up may potentially be safely managed as an outpatient. Initial evaluation and management of PPP usually requires hospitalization, however, because of the risks of suicide, infanticide, and child maltreatment.23
Hospitalization. Mother-infant bonding is important, but safety is paramount if a mother is psychotic—especially if she is experiencing psychotic thoughts about her infant. If possible, the infant should remain with family members during the mother’s hospitalization. Supervised mother-infant visits are often arranged, as appropriate.
Mood-stabilizing medications, including antipsychotics, are mainstays of treatment.24 In some cases, conventional antipsychotics such as haloperidol may be useful because of a lower risk of weight gain or of sedation that could impair a mother’s ability to respond to her infant. Electroconvulsive therapy often yields rapid symptomatic improvement for mothers with postpartum mood or psychotic symptoms.25
Discharge planning. Assuming that the mother adheres to prescribed treatment, discharge may occur within 1 week. Plan discharge arrangements carefully (Table 4).28 A team approach can be very useful within the outpatient clinic. In the model of the Perinatal Psychiatry Clinic of Connections in suburban Cleveland, OH, the mother’s treatment team includes perinatal psychiatrists, nurses, counsellors, case managers (who do home visits), and peer counselors.
Outpatient civil commitment, in which patients are mandated to accept treatment, is an option in some jurisdictions and could help ensure that patients receive treatment consistently.
Table 4
Discharge planning for safety of mother and infant
| Notify child protective services (CPS) depending on the risk to the child. Case-by-case review is needed to assess whether the infant should be removed. CPS may put in place a plan for safety, short of removal. The plan may require that the woman continue psychiatric care |
| Meet with the patient and family to discuss her diagnosis, the risks, the importance of continued medication adherence, and the need for family or social supports to assist with child care |
| Consider engaging visiting nurses or doulas to provide help and support at home |
| Schedule frequent outpatient appointments for the mother after discharge |
| Consider family therapy after the mother has improved because of her risk for affective episodes outside the postpartum28 |
Related resources
- Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26. www.CurrentPsychiatry.com.
- Gentile S. Infant safety with antipsychotic therapy in breastfeeding: a systematic review. J Clin Psychiatry. 2008;69(4):666-673.
- Miller LJ. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing, Inc; 1999.
- Stowe ZN. The use of mood stabilizers during breastfeeding. J Clin Psychiatry. 2007;68(suppl 9):22-28.
- Toxicology Data Network (Toxnet). Literature on reproductive risks associated with psychotropics. National Library of Medicine. http://toxnet.nlm.nih.gov.
- Haloperidol • Haldol
- Lithium • various
- Olanzapine • Zyprexa
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Dr. Resnick, a forensic psychiatrist and coauthor of this article, testified for the defense in both trials of Andrea Yates.
1. Resnick PJ. The Andrea Yates case: insanity on trial. Cleveland State Law Review. 2007;55(2):147-156.
2. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl. 2):29-33.
3. Knops GG. Postpartum mood disorders. Postgrad Med. 1993;93:103-116.
4. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126:73-82.
5. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psychiatry. 2005;162:1578-1587.
6. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. In press.
7. Wisner K, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30:77-87.
8. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behaviour in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
9. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Women’s Health. 2006;15(4):352-368.
11. Attia E, Downey J, Oberman M. Postpartum psychoses. In: Miller LJ, ed. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing Inc.; 1999:99-117.
12. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
13. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
14. Meltzer-Brody S, Payne J, Rubinow D. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
15. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54:21-28.
16. Wisner KL, Gracious BL, Piontek CM, et al. Postpartum disorders: phenomenology, treatment approaches, and relationship to infanticide. In: Spinelli MG, ed. Infanticide: psychosocial and legal perspectives on mothers who kill. Washington, DC: American Psychiatric Publishing, Inc.; 2003.
17. Fairbrother N, Abramowitz JS. New parenthood as a risk factors for the development of obsessional problems. Behav Res Ther. 2007;45(9):2155-2163.
18. Cohen LS, Sichel DA, Robertson LM, et al. Postpartum prophylaxis for women with bipolar disorder. Am J Psychiatry. 1995;152(11):1641-1645.
19. Stewart DE, Klompenhouwer JL, Kendell RE, et al. Prophylactic lithium in puerperal psychosis. Br J Psychiatry. 1991;158:393-397.
20. Sharma V, Smith A, Mazmanian D. Olanzapine in the prevention of postpartum psychosis and mood episodes in bipolar disorder. Bipolar Disord. 2006;8(4):400-404.
21. Pfuhlmann B, Stoeber G, Beckmann H. Postpartum psychoses: prognosis, risk factors, and treatment. Curr Psychiatry Rep. 2002;4(3):185-190.
22. Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord. 2003;5(2):98-105.
23. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
24. Connell M. The postpartum psychosis defense and feminism: more or less justice for women? Case Western Reserve Law Review. 2002;53:143.-
25. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
26. Engqvist I, Nilsson A, Nilsson K, et al. Strategies in caring for women with postpartum psychosis—an interview study with psychiatric nurses. J Clin Nurs. 2007;16(7):1333-1342.
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(4):411-431.
28. Robertson E, Jones I, Haque S, et al. Risk of puerperal and non-puerperal recurrence of illness following bipolar affective puerperal (post-partum) psychosis. Br J Psychiatry. 2005;186:258-259.
In June 2001, Andrea Yates drowned her 5 children ages 6 months to 7 years in the bathtub of their home. She had delusions that her house was bugged and television cameras were monitoring her mothering skills. She came to believe that “the one and only Satan” was within her, and that her children would burn in hell if she did not save their souls while they were still innocent.
Her conviction of capital murder in her first trial was overturned on appeal. She was found not guilty by reason of insanity at her retrial in 2006 and committed to a Texas state mental hospital.1
Postpartum psychosis (PPP) presents dramatically days to weeks after delivery, with wide-ranging symptoms that can include dysphoric mania and delirium. Because untreated PPP has an estimated 4% risk of infanticide (murder of the infant in the first year of life),2 and a 5% risk of suicide,3 psychiatric hospitalization usually is required to protect the mother and her baby.
The diagnosis may be missed, however, because postpartum psychotic symptoms wax and wane and suspiciousness or poor insight cause some women—such as Andrea Yates—to hide their delusional thinking from their families. This article discusses the risk factors, prevention, and treatment of PPP, including a review of:
- infanticide and suicide risks in the postpartum period
- increased susceptibility to PPP in women with bipolar disorder and other psychiatric disorders
- hospitalization for support and safety of the mother and her infant.
Risks of infanticide and suicide
A number of motives exist for infanticide (Table 1).4 Psychiatric literature shows that mothers who kill their children often have experienced psychosis, suicidality, depression, and considerable life stress.5 Common factors include alcohol use, limited social support, and a personal history of abuse. Studies on infanticide found a significant increase in common psychiatric disorders and financial stress among the mothers. Neonaticide (murder of the infant in the first day of life) generally is not related to PPP because PPP usually does not begin until after the day of delivery.6
Among women who develop psychiatric illness, homicidal ideation is more frequent in those with a perinatal onset of psychopathology.7 Infanticidal ideas and behavior are associated with psychotic ideas about the infant.8 Suicide is the cause of up to 20% of postpartum deaths.9
Table 1
Motives for infanticide: Mental illness or something else?
| Motives | Examples |
|---|---|
| Likely related to postpartum psychosis or depression | |
| Altruistic | A depressed or psychotic mother may believe she is sending her baby to heaven to prevent suffering on earth |
| A suicidal mother may kill her infant along with herself rather than leave the child alone | |
| Acutely psychotic | A mother kills her baby for no comprehensible reason, such as in response to command hallucinations or the confusion of delirium |
| Rarely related to postpartum psychosis | |
| Fatal maltreatment | ‘Battered child’ syndrome is the most common cause of infanticide; death often occurs after chronic abuse or neglect |
| A minority of perpetrators are psychotic; a mother out of touch with reality may have difficulty providing for her infant’s needs | |
| Not likely related to postpartum psychosis | |
| Unwanted child | Parent does not want child because of inconvenience or out-of-wedlock birth |
| Spouse revenge | Murder of a child to cause emotional suffering for the other parent is the least frequent motive for infanticide |
| Source: Reference 4 | |
The bipolar connection
Many factors can elevate the risk of PPP, including sleep deprivation in susceptible women, the hormonal shifts after birth, and psychiatric comorbidity (Table 2). Nearly three-fourths (>72%) of mothers with PPP have bipolar disorder or schizoaffective disorder, whereas 12% have schizophrenia.10 Some authors consider PPP to be bipolar disorder until proven otherwise. Mothers with a history of bipolar disorder or PPP have a 100-fold increase in rates of psychiatric hospitalization in the postpartum period.11
Presentation. PPP is relatively rare, occurring at a rate of 1 to 3 cases per 1,000 births. Symptoms often have an abrupt onset, within days to weeks of delivery.10 In at least one-half of cases, symptoms begin by the third postpartum day,13 when many mothers have been discharged home and may be solely responsible for their infants.
Symptoms include confusion, bizarre behaviors, hallucinations (including rarer types such as tactile and olfactory), mood lability (ranging from euphoria to depression), decreased need for sleep or insomnia, restlessness, agitation, disorganized thinking, and bizarre delusions of relatively rapid onset.13 One mother might believe God wants her baby to be sacrificed as the second coming of the Messiah, a second may believe she has special powers, and a third that her baby is defective.
Table 2
Postpartum psychosis: Risk factors supported by evidence
| Sleep deprivation in susceptible women |
| Hormonal shifts after birth (primarily the rapid drop in estrogen) |
| Psychosocial stressors such as marital problems, older age, single motherhood, lower socioeconomic status |
| Bipolar disorder or schizoaffective disorder |
| Past history of postpartum psychosis |
| Family history of postpartum psychosis |
| Previous psychiatric hospitalization, especially during the prenatal period for a bipolar or psychotic condition |
| Menstruation or cessation of lactation |
Obstetric factors that can cause a small increase in relative risk:
|
| Source: For bibliographic citations |
Differential diagnosis
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms (Box).11
Postpartum depression (PPD) occurs in approximately 10% to 15% of new mothers.14 Depressive symptoms occur within weeks to months after delivery and often coexist with anxious symptoms. Some women with severe depression may present with psychotic symptoms. A mother may experience insomnia, sometimes not being able to sleep when the baby is sleeping. She may lack interest in caring for her baby and experience difficulty bonding.
At times it can be difficult to distinguish PPD from PPP. When evaluating a mother who is referred for “postpartum depression,” consider PPP in the differential diagnosis. A woman with PPD or PPP may report depressed mood, but in PPP this symptom usually is related to rapid mood changes. Other clinical features that point toward PPP are abnormal hallucinations (such as olfactory or tactile), hypomanic or mixed mood symptoms, and confusion.
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms, giving special consideration to metabolic, neurologic, cardiovascular, infectious, and substance- or medication-induced origins. The extensive differential diagnosis includes:
- thyroiditis
- tumor
- CNS infection
- head injury
- embolism
- eclampsia
- substance withdrawal
- medication-induced (such as corticosteroids)
- electrolyte anomalies
- anoxia
- vitamin B12 deficiency.11
Psychosis vs OCD. Psychotic thinking and behaviors also must be differentiated from obsessive thoughts and compulsions.10,16 Obsessive compulsive disorder (OCD) may be exacerbated or emerge for the first time during the perinatal period.17
In postpartum OCD, women may experience intrusive thoughts of accidental or purposeful harm to their baby. As opposed to women with PPP, mothers with OCD are not out of touch with reality and their thoughts are ego-dystonic.17 When these mothers have thoughts of their infants being harmed, they realize that these thoughts are not plans but fears and they try to avoid the thoughts.
Preventing PPP
Bipolar disorder is one of the most difficult disorders to treat during pregnancy because the serious risks of untreated illness must be balanced against the potential teratogenic risk of medications. Nevertheless, proactively managing bipolar disorder during pregnancy may reduce the risk of PPP.10
Closely monitor women with a history of bipolar disorder or PPP. During pregnancy, counsel them—and their partners—to:
- anticipate that depressive or psychotic symptoms could develop within days after delivery18
- seek treatment immediately if this occurs.
Postpartum medication. Whether or not a woman with bipolar disorder takes medication during pregnancy, consider treatment with mood stabilizers or atypical antipsychotics in the postpartum to prevent PPP (Table 3). Evidence is limited, but a search of PubMed found 1 study in which prophylactic lithium was given late in the third trimester or immediately after delivery to 21 women with a history of bipolar disorder or PPP. Only 2 patients had a psychotic recurrence while on prophylactic lithium; 1 unexplained stillbirth occurred.19
A retrospective study examined the course of women with bipolar disorder, some of whom were given prophylactic mood stabilizers immediately in the postpartum. One of 14 who received antimanic agents relapsed within the first 3 months postpartum, compared with 8 of 13 who were not so treated.18
Compared with antiepileptics, less information is available about the use of atypical antipsychotics in pregnancy and lactation. Antipsychotics’ potential advantage in women at risk for PPP is that these agents may help prevent or treat both manic and psychotic symptoms.
In a small, naturalistic, prospective study, 11 women at risk for PPP received olanzapine alone or with an antidepressant or mood stabilizer for at least 4 weeks after delivery. Two (18%) experienced a postpartum mood episode, compared with 8 (57%) of 14 other at-risk women who received antidepressants, mood stabilizers, or no medication.20
When you discuss breast-feeding, consider possible risks to the neonate as well as potential sleep interruption for the mother. If a mother has a supportive partner, the partner might be put in charge of night-time feedings in a routine combining breast-feeding and bottle-feeding. In some cases you may need to recommend cessation of lactation.21
Table 3
Treating postpartum psychosis: Consider 3 components
| Component | Recommendations |
|---|---|
| Hospitalization vs home care | Hospitalize in most cases because of emergent severe symptoms and fluctuating course; base decision on risk evaluation/safety issues for patient and infant After discharge, visiting nurses are useful to help monitor the mother’s condition at home |
| Psychoeducation | Educate patient, family, and social support network; address risks to mother and infant and risks in future pregnancies |
| Medication | When prescribing mood stabilizers and/or antipsychotics, consider:
|
Managing PPP
Early symptoms. Because of its severity and rapid evolution, PPP often presents as a psychiatric emergency. Monitor atrisk patients’ sleep patterns and mood for early signs of psychosis.22 Watch especially for hypomanic symptoms such as elevated or mixed mood and decreased judgment, which are common early in PPP.13
A mother with few signs of abnormal mood, good social support, and close follow-up may potentially be safely managed as an outpatient. Initial evaluation and management of PPP usually requires hospitalization, however, because of the risks of suicide, infanticide, and child maltreatment.23
Hospitalization. Mother-infant bonding is important, but safety is paramount if a mother is psychotic—especially if she is experiencing psychotic thoughts about her infant. If possible, the infant should remain with family members during the mother’s hospitalization. Supervised mother-infant visits are often arranged, as appropriate.
Mood-stabilizing medications, including antipsychotics, are mainstays of treatment.24 In some cases, conventional antipsychotics such as haloperidol may be useful because of a lower risk of weight gain or of sedation that could impair a mother’s ability to respond to her infant. Electroconvulsive therapy often yields rapid symptomatic improvement for mothers with postpartum mood or psychotic symptoms.25
Discharge planning. Assuming that the mother adheres to prescribed treatment, discharge may occur within 1 week. Plan discharge arrangements carefully (Table 4).28 A team approach can be very useful within the outpatient clinic. In the model of the Perinatal Psychiatry Clinic of Connections in suburban Cleveland, OH, the mother’s treatment team includes perinatal psychiatrists, nurses, counsellors, case managers (who do home visits), and peer counselors.
Outpatient civil commitment, in which patients are mandated to accept treatment, is an option in some jurisdictions and could help ensure that patients receive treatment consistently.
Table 4
Discharge planning for safety of mother and infant
| Notify child protective services (CPS) depending on the risk to the child. Case-by-case review is needed to assess whether the infant should be removed. CPS may put in place a plan for safety, short of removal. The plan may require that the woman continue psychiatric care |
| Meet with the patient and family to discuss her diagnosis, the risks, the importance of continued medication adherence, and the need for family or social supports to assist with child care |
| Consider engaging visiting nurses or doulas to provide help and support at home |
| Schedule frequent outpatient appointments for the mother after discharge |
| Consider family therapy after the mother has improved because of her risk for affective episodes outside the postpartum28 |
Related resources
- Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26. www.CurrentPsychiatry.com.
- Gentile S. Infant safety with antipsychotic therapy in breastfeeding: a systematic review. J Clin Psychiatry. 2008;69(4):666-673.
- Miller LJ. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing, Inc; 1999.
- Stowe ZN. The use of mood stabilizers during breastfeeding. J Clin Psychiatry. 2007;68(suppl 9):22-28.
- Toxicology Data Network (Toxnet). Literature on reproductive risks associated with psychotropics. National Library of Medicine. http://toxnet.nlm.nih.gov.
- Haloperidol • Haldol
- Lithium • various
- Olanzapine • Zyprexa
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Dr. Resnick, a forensic psychiatrist and coauthor of this article, testified for the defense in both trials of Andrea Yates.
In June 2001, Andrea Yates drowned her 5 children ages 6 months to 7 years in the bathtub of their home. She had delusions that her house was bugged and television cameras were monitoring her mothering skills. She came to believe that “the one and only Satan” was within her, and that her children would burn in hell if she did not save their souls while they were still innocent.
Her conviction of capital murder in her first trial was overturned on appeal. She was found not guilty by reason of insanity at her retrial in 2006 and committed to a Texas state mental hospital.1
Postpartum psychosis (PPP) presents dramatically days to weeks after delivery, with wide-ranging symptoms that can include dysphoric mania and delirium. Because untreated PPP has an estimated 4% risk of infanticide (murder of the infant in the first year of life),2 and a 5% risk of suicide,3 psychiatric hospitalization usually is required to protect the mother and her baby.
The diagnosis may be missed, however, because postpartum psychotic symptoms wax and wane and suspiciousness or poor insight cause some women—such as Andrea Yates—to hide their delusional thinking from their families. This article discusses the risk factors, prevention, and treatment of PPP, including a review of:
- infanticide and suicide risks in the postpartum period
- increased susceptibility to PPP in women with bipolar disorder and other psychiatric disorders
- hospitalization for support and safety of the mother and her infant.
Risks of infanticide and suicide
A number of motives exist for infanticide (Table 1).4 Psychiatric literature shows that mothers who kill their children often have experienced psychosis, suicidality, depression, and considerable life stress.5 Common factors include alcohol use, limited social support, and a personal history of abuse. Studies on infanticide found a significant increase in common psychiatric disorders and financial stress among the mothers. Neonaticide (murder of the infant in the first day of life) generally is not related to PPP because PPP usually does not begin until after the day of delivery.6
Among women who develop psychiatric illness, homicidal ideation is more frequent in those with a perinatal onset of psychopathology.7 Infanticidal ideas and behavior are associated with psychotic ideas about the infant.8 Suicide is the cause of up to 20% of postpartum deaths.9
Table 1
Motives for infanticide: Mental illness or something else?
| Motives | Examples |
|---|---|
| Likely related to postpartum psychosis or depression | |
| Altruistic | A depressed or psychotic mother may believe she is sending her baby to heaven to prevent suffering on earth |
| A suicidal mother may kill her infant along with herself rather than leave the child alone | |
| Acutely psychotic | A mother kills her baby for no comprehensible reason, such as in response to command hallucinations or the confusion of delirium |
| Rarely related to postpartum psychosis | |
| Fatal maltreatment | ‘Battered child’ syndrome is the most common cause of infanticide; death often occurs after chronic abuse or neglect |
| A minority of perpetrators are psychotic; a mother out of touch with reality may have difficulty providing for her infant’s needs | |
| Not likely related to postpartum psychosis | |
| Unwanted child | Parent does not want child because of inconvenience or out-of-wedlock birth |
| Spouse revenge | Murder of a child to cause emotional suffering for the other parent is the least frequent motive for infanticide |
| Source: Reference 4 | |
The bipolar connection
Many factors can elevate the risk of PPP, including sleep deprivation in susceptible women, the hormonal shifts after birth, and psychiatric comorbidity (Table 2). Nearly three-fourths (>72%) of mothers with PPP have bipolar disorder or schizoaffective disorder, whereas 12% have schizophrenia.10 Some authors consider PPP to be bipolar disorder until proven otherwise. Mothers with a history of bipolar disorder or PPP have a 100-fold increase in rates of psychiatric hospitalization in the postpartum period.11
Presentation. PPP is relatively rare, occurring at a rate of 1 to 3 cases per 1,000 births. Symptoms often have an abrupt onset, within days to weeks of delivery.10 In at least one-half of cases, symptoms begin by the third postpartum day,13 when many mothers have been discharged home and may be solely responsible for their infants.
Symptoms include confusion, bizarre behaviors, hallucinations (including rarer types such as tactile and olfactory), mood lability (ranging from euphoria to depression), decreased need for sleep or insomnia, restlessness, agitation, disorganized thinking, and bizarre delusions of relatively rapid onset.13 One mother might believe God wants her baby to be sacrificed as the second coming of the Messiah, a second may believe she has special powers, and a third that her baby is defective.
Table 2
Postpartum psychosis: Risk factors supported by evidence
| Sleep deprivation in susceptible women |
| Hormonal shifts after birth (primarily the rapid drop in estrogen) |
| Psychosocial stressors such as marital problems, older age, single motherhood, lower socioeconomic status |
| Bipolar disorder or schizoaffective disorder |
| Past history of postpartum psychosis |
| Family history of postpartum psychosis |
| Previous psychiatric hospitalization, especially during the prenatal period for a bipolar or psychotic condition |
| Menstruation or cessation of lactation |
Obstetric factors that can cause a small increase in relative risk:
|
| Source: For bibliographic citations |
Differential diagnosis
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms (Box).11
Postpartum depression (PPD) occurs in approximately 10% to 15% of new mothers.14 Depressive symptoms occur within weeks to months after delivery and often coexist with anxious symptoms. Some women with severe depression may present with psychotic symptoms. A mother may experience insomnia, sometimes not being able to sleep when the baby is sleeping. She may lack interest in caring for her baby and experience difficulty bonding.
At times it can be difficult to distinguish PPD from PPP. When evaluating a mother who is referred for “postpartum depression,” consider PPP in the differential diagnosis. A woman with PPD or PPP may report depressed mood, but in PPP this symptom usually is related to rapid mood changes. Other clinical features that point toward PPP are abnormal hallucinations (such as olfactory or tactile), hypomanic or mixed mood symptoms, and confusion.
When evaluating a postpartum woman with psychotic symptoms, stay in contact with her obstetrician and the child’s pediatrician. Rule out delirium and organic causes of the mother’s symptoms, giving special consideration to metabolic, neurologic, cardiovascular, infectious, and substance- or medication-induced origins. The extensive differential diagnosis includes:
- thyroiditis
- tumor
- CNS infection
- head injury
- embolism
- eclampsia
- substance withdrawal
- medication-induced (such as corticosteroids)
- electrolyte anomalies
- anoxia
- vitamin B12 deficiency.11
Psychosis vs OCD. Psychotic thinking and behaviors also must be differentiated from obsessive thoughts and compulsions.10,16 Obsessive compulsive disorder (OCD) may be exacerbated or emerge for the first time during the perinatal period.17
In postpartum OCD, women may experience intrusive thoughts of accidental or purposeful harm to their baby. As opposed to women with PPP, mothers with OCD are not out of touch with reality and their thoughts are ego-dystonic.17 When these mothers have thoughts of their infants being harmed, they realize that these thoughts are not plans but fears and they try to avoid the thoughts.
Preventing PPP
Bipolar disorder is one of the most difficult disorders to treat during pregnancy because the serious risks of untreated illness must be balanced against the potential teratogenic risk of medications. Nevertheless, proactively managing bipolar disorder during pregnancy may reduce the risk of PPP.10
Closely monitor women with a history of bipolar disorder or PPP. During pregnancy, counsel them—and their partners—to:
- anticipate that depressive or psychotic symptoms could develop within days after delivery18
- seek treatment immediately if this occurs.
Postpartum medication. Whether or not a woman with bipolar disorder takes medication during pregnancy, consider treatment with mood stabilizers or atypical antipsychotics in the postpartum to prevent PPP (Table 3). Evidence is limited, but a search of PubMed found 1 study in which prophylactic lithium was given late in the third trimester or immediately after delivery to 21 women with a history of bipolar disorder or PPP. Only 2 patients had a psychotic recurrence while on prophylactic lithium; 1 unexplained stillbirth occurred.19
A retrospective study examined the course of women with bipolar disorder, some of whom were given prophylactic mood stabilizers immediately in the postpartum. One of 14 who received antimanic agents relapsed within the first 3 months postpartum, compared with 8 of 13 who were not so treated.18
Compared with antiepileptics, less information is available about the use of atypical antipsychotics in pregnancy and lactation. Antipsychotics’ potential advantage in women at risk for PPP is that these agents may help prevent or treat both manic and psychotic symptoms.
In a small, naturalistic, prospective study, 11 women at risk for PPP received olanzapine alone or with an antidepressant or mood stabilizer for at least 4 weeks after delivery. Two (18%) experienced a postpartum mood episode, compared with 8 (57%) of 14 other at-risk women who received antidepressants, mood stabilizers, or no medication.20
When you discuss breast-feeding, consider possible risks to the neonate as well as potential sleep interruption for the mother. If a mother has a supportive partner, the partner might be put in charge of night-time feedings in a routine combining breast-feeding and bottle-feeding. In some cases you may need to recommend cessation of lactation.21
Table 3
Treating postpartum psychosis: Consider 3 components
| Component | Recommendations |
|---|---|
| Hospitalization vs home care | Hospitalize in most cases because of emergent severe symptoms and fluctuating course; base decision on risk evaluation/safety issues for patient and infant After discharge, visiting nurses are useful to help monitor the mother’s condition at home |
| Psychoeducation | Educate patient, family, and social support network; address risks to mother and infant and risks in future pregnancies |
| Medication | When prescribing mood stabilizers and/or antipsychotics, consider:
|
Managing PPP
Early symptoms. Because of its severity and rapid evolution, PPP often presents as a psychiatric emergency. Monitor atrisk patients’ sleep patterns and mood for early signs of psychosis.22 Watch especially for hypomanic symptoms such as elevated or mixed mood and decreased judgment, which are common early in PPP.13
A mother with few signs of abnormal mood, good social support, and close follow-up may potentially be safely managed as an outpatient. Initial evaluation and management of PPP usually requires hospitalization, however, because of the risks of suicide, infanticide, and child maltreatment.23
Hospitalization. Mother-infant bonding is important, but safety is paramount if a mother is psychotic—especially if she is experiencing psychotic thoughts about her infant. If possible, the infant should remain with family members during the mother’s hospitalization. Supervised mother-infant visits are often arranged, as appropriate.
Mood-stabilizing medications, including antipsychotics, are mainstays of treatment.24 In some cases, conventional antipsychotics such as haloperidol may be useful because of a lower risk of weight gain or of sedation that could impair a mother’s ability to respond to her infant. Electroconvulsive therapy often yields rapid symptomatic improvement for mothers with postpartum mood or psychotic symptoms.25
Discharge planning. Assuming that the mother adheres to prescribed treatment, discharge may occur within 1 week. Plan discharge arrangements carefully (Table 4).28 A team approach can be very useful within the outpatient clinic. In the model of the Perinatal Psychiatry Clinic of Connections in suburban Cleveland, OH, the mother’s treatment team includes perinatal psychiatrists, nurses, counsellors, case managers (who do home visits), and peer counselors.
Outpatient civil commitment, in which patients are mandated to accept treatment, is an option in some jurisdictions and could help ensure that patients receive treatment consistently.
Table 4
Discharge planning for safety of mother and infant
| Notify child protective services (CPS) depending on the risk to the child. Case-by-case review is needed to assess whether the infant should be removed. CPS may put in place a plan for safety, short of removal. The plan may require that the woman continue psychiatric care |
| Meet with the patient and family to discuss her diagnosis, the risks, the importance of continued medication adherence, and the need for family or social supports to assist with child care |
| Consider engaging visiting nurses or doulas to provide help and support at home |
| Schedule frequent outpatient appointments for the mother after discharge |
| Consider family therapy after the mother has improved because of her risk for affective episodes outside the postpartum28 |
Related resources
- Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26. www.CurrentPsychiatry.com.
- Gentile S. Infant safety with antipsychotic therapy in breastfeeding: a systematic review. J Clin Psychiatry. 2008;69(4):666-673.
- Miller LJ. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing, Inc; 1999.
- Stowe ZN. The use of mood stabilizers during breastfeeding. J Clin Psychiatry. 2007;68(suppl 9):22-28.
- Toxicology Data Network (Toxnet). Literature on reproductive risks associated with psychotropics. National Library of Medicine. http://toxnet.nlm.nih.gov.
- Haloperidol • Haldol
- Lithium • various
- Olanzapine • Zyprexa
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Dr. Resnick, a forensic psychiatrist and coauthor of this article, testified for the defense in both trials of Andrea Yates.
1. Resnick PJ. The Andrea Yates case: insanity on trial. Cleveland State Law Review. 2007;55(2):147-156.
2. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl. 2):29-33.
3. Knops GG. Postpartum mood disorders. Postgrad Med. 1993;93:103-116.
4. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126:73-82.
5. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psychiatry. 2005;162:1578-1587.
6. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. In press.
7. Wisner K, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30:77-87.
8. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behaviour in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
9. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Women’s Health. 2006;15(4):352-368.
11. Attia E, Downey J, Oberman M. Postpartum psychoses. In: Miller LJ, ed. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing Inc.; 1999:99-117.
12. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
13. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
14. Meltzer-Brody S, Payne J, Rubinow D. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
15. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54:21-28.
16. Wisner KL, Gracious BL, Piontek CM, et al. Postpartum disorders: phenomenology, treatment approaches, and relationship to infanticide. In: Spinelli MG, ed. Infanticide: psychosocial and legal perspectives on mothers who kill. Washington, DC: American Psychiatric Publishing, Inc.; 2003.
17. Fairbrother N, Abramowitz JS. New parenthood as a risk factors for the development of obsessional problems. Behav Res Ther. 2007;45(9):2155-2163.
18. Cohen LS, Sichel DA, Robertson LM, et al. Postpartum prophylaxis for women with bipolar disorder. Am J Psychiatry. 1995;152(11):1641-1645.
19. Stewart DE, Klompenhouwer JL, Kendell RE, et al. Prophylactic lithium in puerperal psychosis. Br J Psychiatry. 1991;158:393-397.
20. Sharma V, Smith A, Mazmanian D. Olanzapine in the prevention of postpartum psychosis and mood episodes in bipolar disorder. Bipolar Disord. 2006;8(4):400-404.
21. Pfuhlmann B, Stoeber G, Beckmann H. Postpartum psychoses: prognosis, risk factors, and treatment. Curr Psychiatry Rep. 2002;4(3):185-190.
22. Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord. 2003;5(2):98-105.
23. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
24. Connell M. The postpartum psychosis defense and feminism: more or less justice for women? Case Western Reserve Law Review. 2002;53:143.-
25. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
26. Engqvist I, Nilsson A, Nilsson K, et al. Strategies in caring for women with postpartum psychosis—an interview study with psychiatric nurses. J Clin Nurs. 2007;16(7):1333-1342.
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(4):411-431.
28. Robertson E, Jones I, Haque S, et al. Risk of puerperal and non-puerperal recurrence of illness following bipolar affective puerperal (post-partum) psychosis. Br J Psychiatry. 2005;186:258-259.
1. Resnick PJ. The Andrea Yates case: insanity on trial. Cleveland State Law Review. 2007;55(2):147-156.
2. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl. 2):29-33.
3. Knops GG. Postpartum mood disorders. Postgrad Med. 1993;93:103-116.
4. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126:73-82.
5. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psychiatry. 2005;162:1578-1587.
6. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. In press.
7. Wisner K, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30:77-87.
8. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behaviour in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
9. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Women’s Health. 2006;15(4):352-368.
11. Attia E, Downey J, Oberman M. Postpartum psychoses. In: Miller LJ, ed. Postpartum mood disorders. Washington, DC: American Psychiatric Publishing Inc.; 1999:99-117.
12. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
13. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
14. Meltzer-Brody S, Payne J, Rubinow D. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
15. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54:21-28.
16. Wisner KL, Gracious BL, Piontek CM, et al. Postpartum disorders: phenomenology, treatment approaches, and relationship to infanticide. In: Spinelli MG, ed. Infanticide: psychosocial and legal perspectives on mothers who kill. Washington, DC: American Psychiatric Publishing, Inc.; 2003.
17. Fairbrother N, Abramowitz JS. New parenthood as a risk factors for the development of obsessional problems. Behav Res Ther. 2007;45(9):2155-2163.
18. Cohen LS, Sichel DA, Robertson LM, et al. Postpartum prophylaxis for women with bipolar disorder. Am J Psychiatry. 1995;152(11):1641-1645.
19. Stewart DE, Klompenhouwer JL, Kendell RE, et al. Prophylactic lithium in puerperal psychosis. Br J Psychiatry. 1991;158:393-397.
20. Sharma V, Smith A, Mazmanian D. Olanzapine in the prevention of postpartum psychosis and mood episodes in bipolar disorder. Bipolar Disord. 2006;8(4):400-404.
21. Pfuhlmann B, Stoeber G, Beckmann H. Postpartum psychoses: prognosis, risk factors, and treatment. Curr Psychiatry Rep. 2002;4(3):185-190.
22. Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord. 2003;5(2):98-105.
23. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
24. Connell M. The postpartum psychosis defense and feminism: more or less justice for women? Case Western Reserve Law Review. 2002;53:143.-
25. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
26. Engqvist I, Nilsson A, Nilsson K, et al. Strategies in caring for women with postpartum psychosis—an interview study with psychiatric nurses. J Clin Nurs. 2007;16(7):1333-1342.
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(4):411-431.
28. Robertson E, Jones I, Haque S, et al. Risk of puerperal and non-puerperal recurrence of illness following bipolar affective puerperal (post-partum) psychosis. Br J Psychiatry. 2005;186:258-259.