User login
Syracuse Hemoglobinopathy Presenting With Tophaceous Gout: A Case Report
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
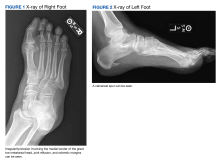
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y