User login
5 Points on Pyogenic Flexor Tenosynovitis of the Hand
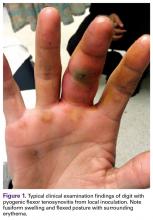
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
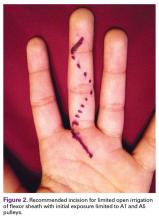
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
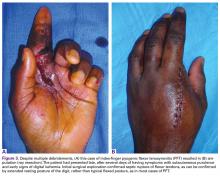
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.