User login
Things We Do for No Reason™: Routine Use of Corticosteroids for the Treatment of Anaphylaxis
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11
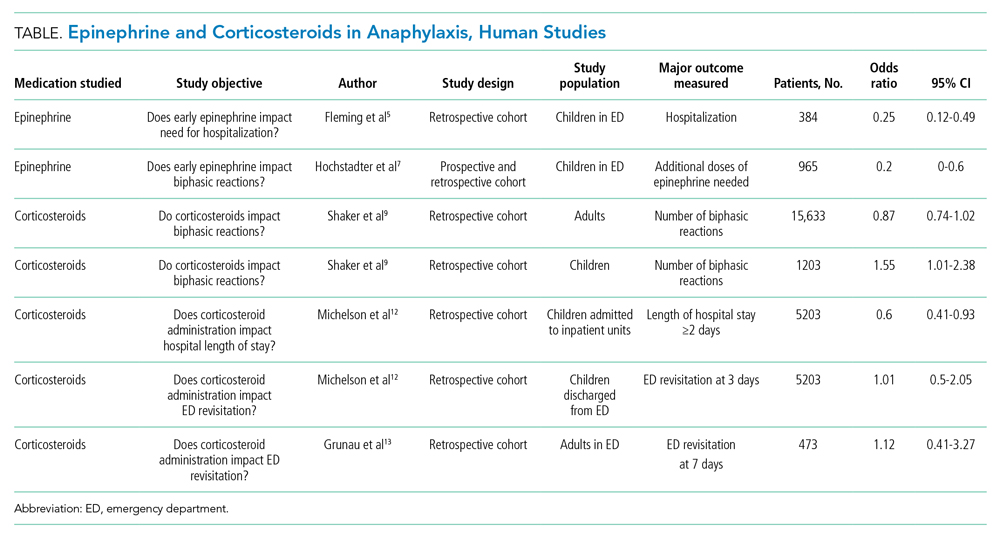
The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
© 2021 Society of Hospital Medicine