User login
Substance use disorders in adolescents with psychiatric comorbidity: When to screen and how to treat
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
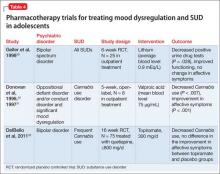
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
Substances use during adolescence is common in the United States. Data from the 2014 Monitoring the Future Survey estimated that among 12th graders, 60.2% used alcohol, 35.1% used marijuana, and 13.9% used a prescription drug for nonmedical use within the previous year.1 An estimated 11.4% of adolescents meet DSM-IV threshold criteria for a substance use disorder (SUD).2 Substance use in adolescents often co-occurs with psychological distress and psychiatric illness. Adolescents with a psychiatric disorder are at increased risk for developing a SUD; conversely, high rates of psychiatric illness are seen in adolescents with a SUD.3,4 In one study, 82% of adolescents hospitalized for SUD treatment were found to have a co-occurring axis I disorder.5 Furthermore, co-occurring psychiatric illness and SUD complicates treatment course and prognosis. Adolescents with co-occurring psychiatric illness and SUD often benefit from an integrated, multimodal treatment approach that includes psychotherapy, pharmacologic interventions, family involvement, and collaboration with community supports.
In this article, we focus on pharmacologic management of non-nicotinic SUDs in adolescents, with an emphasis on those with comorbid psychiatric illness.
Screening and assessment of substance use
It is important to counsel children with a psychiatric illness and their parents about the increased risk for SUD before a patient transitions to adolescence. Discussions about substance abuse should begin during the 5th grade because data suggests that adolescent substance use often starts in middle school (6th to 9th grade). Clinicians should routinely screen adolescent patients for substance use. Nonproprietary screening tools available through the National Institute on Alcohol and Alcoholism and the National Institute on Drug Abuse are listed in Table 1.6-8 The Screening to Brief Intervention (S2BI) is a newer tool that has been shown to be highly effective in identifying adolescents at risk for substance abuse and differentiating severity of illness.8 The S2BI includes screening questions that assess for use of 8 substances in the past year.
Adolescents with psychiatric illness who are identified to be at risk for problems associated with substance use should be evaluated further for the presence or absence of a SUD. The number of criteria a patient endorses over the past year (Table 29) is used to assess SUD severity—mild, moderate, or severe. Additional considerations include substance use patterns such as type, site, quantity, frequency, context, and combinations of substances.
It is important to be curious and nonjudgmental when evaluating substance use patterns with adolescents to obtain a comprehensive assessment. Teenagers often are creative and inventive in their efforts to maximize intoxication, which can put them at risk for complications associated with acute intoxication. Rapidly evolving methods of ingesting highly concentrated forms of tetrahydrocannabinol (“wax,” “dabs”) are an example of use patterns that are ahead of what is reported in the literature.
Any substance use in an adolescent with a psychiatric illness is of concern and should be monitored closely because of the potential impact of substance use on the co-occurring psychiatric illness and possible interactions between the abused substance and prescribed medication.
Treatment interventions
Although this review will focus on pharmacotherapy, individual, group, and family psychotherapies are a critical part of a treatment plan for adolescents with comorbid psychiatric illness and SUD (Table 3). Collaboration with community supports, including school and legal officials, can help reinforce contingencies and assist with connecting a teen with positive prosocial activities. Involvement with mutual help organizations, such as Alcoholics Anonymous, can facilitate adolescent engagement with a positive sober network.10
Pharmacologic strategies for treating co-occurring psychiatric illness and SUD include medication to:
• decrease substance use and promote abstinence
• alleviate withdrawal symptoms (medication to treat withdrawal symptoms and agonist treatments)
• block the effect of substance use (antagonist agents)
• decrease likelihood of substance use with aversive agents
• target comorbid psychiatric illness.
Medication to decrease substance use and promote abstinence. One strategy is to target cravings and urges to use substances with medication. Naltrexone is an opiate antagonist FDA-approved for treating alcohol and opioid use disorders in adults and is available as a daily oral medication and a monthly injectable depot preparation (extended-release naltrexone). Two small open-label studies showed decreased alcohol use with naltrexone treatment in adolescents with alcohol use disorder.11,12 In a randomized double-blind placebo controlled (RCT) crossover study of 22 adolescent problem drinkers, naltrexone, 50 mg/d, reduced the likelihood of drinking and heavy drinking (P ≤ .03).13 Acamprosate, another anti-craving medication FDA-approved for treating alcohol use disorder in adults, has no data on the safety or efficacy for adolescent alcohol use disorder.
There is limited research on agents that decrease use and promote abstinence from non-nicotinic substances other than alcohol. There is one pilot RCT that evaluated N-acetylcysteine (NAC)—an over-the-counter supplement that modulates the glutamate system—for treating adolescent Cannabis dependence. Treatment with NAC, 2,400 mg/d, was well tolerated and had twice the odds of increasing negative urine cannabinoid tests during treatment than placebo.14 Although NAC treatment was associated with decreased Cannabis use, it did not significantly decrease cravings compared with placebo.15
Medication to alleviate withdrawal symptoms. Some patients may find the physical discomfort and psychological distress associated with substance withdrawal so intolerable that to avoid it they continue to use drugs or alcohol. Medication to treat withdrawal symptoms and agonist treatments can be used to alleviate discomfort and distress associated with withdrawal. Agonist treatments, such as methadone and buprenorphine, bind to the same receptors as the target substance, which allows the patient to shift to controlled use of a prescribed substitute. Agonist treatments are used for short detoxification and over longer periods of time for maintenance treatment. Methadone, which decreases craving and withdrawal symptoms from opiates by binding to the μ-opiate receptor and blocking other substances from binding, is frequently used for detoxification and maintenance treatment in adults. There is limited data on methadone substitution therapy for adolescents in the United States.16 Methadone maintenance for adolescents in the United States is restricted to severe cases of opioid use disorder. Federal guidelines specify that adolescents age <18 can only receive methadone if they have had 2 unsuccessful detoxification attempts or outpatient psychosocial treatments and have met DSM criteria for an opioid use disorder for 1 year.17
Buprenorphine is a partial μ-opiate receptor agonist that is FDA-approved for use in adolescents age ≥16 with opioid dependence. Although a waiver from the U.S. Drug Enforcement Administration is required to prescribe buprenorphine, it generally can be administered in outpatient settings with relative ease compared with methadone.
Marsch et al18 examined the efficacy of buprenorphine compared with clonidine for detoxification over 1 month in 36 adolescents with opioid dependence. Clonidine is an α-2 adrenergic agonist that often is used during detoxification from opioids.19 Although both buprenorphine and clonidine relieved withdrawal symptoms, a significantly higher percentage of patients receiving buprenorphine completed treatment (72%) compared with those taking clonidine (39%) (P < .05).18 Detoxification with buprenorphine also was associated with a higher percentage of negative urine drug screens (64% vs 32%, P = .01), and those receiving buprenorphine were more likely to continue on naltrexone maintenance for continued medication-assisted treatment after detoxification compared with those randomized to clonidine.
Woody et al20 compared use of buprenorphine/naloxone for opioid detoxification vs short-term maintenance. Patients age 16 to 21 were randomized to detoxification over 2 weeks vs stabilization and maintenance for 9 weeks and taper over 3 weeks. Maintenance treatment with buprenorphine/naloxone was associated with less opioid use, less injection drug use, and less need for addiction treatment outside of that received through the study compared with detoxification treatment. When buprenorphine/naloxone was discontinued both the detoxification and maintenance groups had high rates of positive urine toxicology screens at 1-year follow up (mean 48% to 72%). These data suggests maintenance with buprenorphine/ naloxone for adolescents and young adults is more effective than short-term detoxification for stabilizing opioid use disorders, although optimal treatment duration is unclear. Clinically, it is important to continue buprenorphine/naloxone maintenance until the patient has stabilized in recovery and has acquired coping skills to manage urges, cravings, and psychological distress (eg, anger, stress) that often arise during a slow taper of agonist treatment.
Antagonist treatment to block the effect of substance use
As an opioid receptor antagonist, naltrexone is effective for treating opioid use disorder because it blocks the action of opioids. Fishman et al21 published a descriptive series of 16 adolescents and young adults followed over 4 months who received the injectable depot preparation (extended-release) naltrexone while in residential treatment, and then discharged to outpatient care. Most patients who received extended-release naltrexone remained in outpatient treatment (63%) and reduced their opioid use or were abstinent at 4 months (56%). One barrier to naltrexone treatment is the need to be abstinent from opioids for 7 to 10 days to prevent precipitated opioid withdrawal. Therefore, naltrexone is a good option for adolescents who present for treatment early and are not physiologically dependent on opioids or are receiving treatment in a structured environment after detoxification, such as residential treatment or sober living.
Aversive agents to diminish substance use. Aversive agents produce an unpleasant reaction when a target substance is consumed. Disulfiram is prototypic aversive agent that prevents the breakdown of acetaldehyde, a toxic metabolite of alcohol. Patients who drink alcohol while taking disulfiram may experience adverse effects, including tachycardia, shortness of breath, nausea, dizziness, and confusion. There have been 2 studies examining the efficacy of disulfiram in adolescents with alcohol use disorder. Niederhofer et al22 found that disulfiram treatment significantly increased cumulative abstinence in a small RCT (P = .012). In another small randomized, open-label, 3-month study of adolescents who received disulfiram or naltrexone in addition to weekly psychotherapy, disulfiram was superior to naltrexone in mean days abstinent from alcohol, 84 days vs 51 days, respectively (P = .0001).23 Often adolescents are not willing to adhere to disulfiram because they are concerned about the aversive reaction when combined with alcohol use. Consider prescribing disulfiram for adolescents who are about to go “on pass” from a therapeutic school or residential SUD treatment center and will be returning to an environment where they may be tempted to use alcohol.
Pharmacotherapy to treat co-occurring psychiatric illness
Continued treatment of a psychiatric illness that co-occurs with SUD is important. As we recommended, consider psychosocial treatments for both the SUD and comorbid psychopathology. Several single-site RCTs have evaluated the efficacy of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline for depressive disorders in adolescents with a co-occurring SUD.24-28 Most studies have shown improvement in depressive symptoms and substance use in medication and placebo groups.24,25,27,28 However, treatment with fluoxetine, 20 mg/d, or sertraline, 100 mg/d, when compared with placebo was associated with improved depressive symptoms in 1 of 3 studies and had no significant difference in SUD outcome. The authors of these studies believe that the general improvement in depression and the SUD was related to use of cognitive-behavioral therapy (CBT) and/or motivational enhancement therapy.24,25,27,28
Research on the use of mood stabilizers for adolescents with mood dysregulation and a SUD is limited but has suggested benefit associated with pharmacotherapy (Table 4).29-32 Two RCTs and 1 open-label study demonstrated reductions in substance use with mood stabilizer treatment in adolescents with co-occurring SUD and mood dysregulation.29-32 The effect of pharmacotherapy on mood dysregulation ratings are less clear because there was no change in severity of affective symptoms observed in a small RCT of lithium (average blood level 0.9 mEq/L)29; and improvement in affective symptoms was noted in topiramate (300 mg/d) and placebo groups when both groups were treated with concurrent quetiapine.32 Because of the high risk of SUD and severe morbidity in juvenile bipolar disorder and severe mood dysregulation,33 larger RCTs are warranted.
Several studies have evaluated the impact of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder (ADHD) in adolescents with a co-occurring SUD.34-39 The largest and only multisite study evaluated the efficacy of osmotic (extended) release methylphenidate (OROS-MPH) vs placebo for adolescents who also were receiving CBT for SUD.36 In this 16-week RCT, the OROS-MPH and placebo groups showed improvement in self-reported ADHD symptoms with no difference between groups. Parent report of ADHD symptoms did indicate a greater reduction in symptoms in the OROS-MPH group compared with placebo. Both groups had a decrease in self-reported days of substance use over the past month with no differences between groups. Pharmacotherapy trials for ADHD that have included psychotherapy highlight the effectiveness of CBT for SUD and co-occurring psychiatric illness.36,39,40
Although conduct disorder and anxiety disorders commonly co-occur with SUD, there has been less research evaluating the impact of pharmacotherapy on treating these disorders. Riggs et al25,34,35,41 evaluated the impact of pharmacotherapy targeted to co-occurring ADHD and major depressive disorder in the context of conduct disorder and SUD. When evaluated in an outpatient setting, the presence of a treatment intervention to address the co-occurring SUD was an important component that led to a reduction in conduct symptoms.25,35 There have been no comprehensive studies on the impact of pharmacotherapy for treating anxiety and SUD in adolescents.
Recommendations for clinical management
Although more research is needed to evaluate the role of pharmacotherapy for adolescents with co-occurring psychiatric illness and a SUD, recommended practice is to continue pharmacotherapy and closely monitor response to treatment when at-risk substance use begins in patients with co-occurring psychiatric illness. In adolescents with a threshold SUD, continue pharmacotherapy for unstable mood disorders with first-line choices of SSRIs for unipolar depression and second-generation antipsychotics for bipolar spectrum illness. Suggested conservative pharmacological interventions for anxiety disorders include SSRIs and buspirone, which have been shown to be effective for treating anxiety in children and adolescents.42,43 For patients with comorbid ADHD and SUD, if possible, it is recommended to first stabilize substance use (low-level use or abstinence) and consider treating ADHD immediately thereafter with a nonstimulant such as atomoxetine, which has data on efficacy and safety in context to substance use; and/or an α-agonist or an extended-release stimulant. Because of the potential for misuse and toxicity associated with concurrent substance use, benzodiazepines should be considered a last treatment of choice for adolescents with anxiety disorders and a SUD. Similarly, the use of immediate-release stimulants should be avoided in patients with ADHD and a SUD. When prescribing medications that could be misused or toxic when combined with a substance, it is important to evaluate the risk and benefit of continued use of a particular medication and consider prescribing lower quantities to decrease risk for misuse (1- to 2-week supply). Adolescents often are reluctant to engage in SUD treatment and one strategy to consider is to make continued prescription of any medication contingent on engaging in SUD treatment. Enlist parents in helping to monitor, store, and administer their child’s medication to improve adherence and decrease the potential for misuse, diversion, and complications associated with substance intoxication.
Bottom Line
It is important to screen for substance use in adolescents with co-occurring
psychiatric illness and vice versa. When at-risk or hazardous substance use is
detected there are effective psychosocial and pharmacologic interventions that
can be used to treat adolescent substance use disorders alone and in combination
with certain psychiatric disorders.
Related Resources
• National Institute on Drug Abuse. www.drugabuse.gov.
• National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
• Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
Drug Brand Names
Acamprosate • Campral
Atomoxetine • Strattera
Buprenorphine• Subutex
Buprenorphine/naloxone • Suboxone
Buspirone • Buspar
Clonidine • Catapres
Disulfiram • Antabuse
Fluoxetine • Prozac
Lithium • Lithobid, Eskalith
Methadone • Dolophine
Naltrexone • ReVia, Vivitrol
Osmotic (extended) release methylphenidate • Concerta
Sertraline • Zoloft
Topiramate • Topamax
Quetiapine • Seroquel
Valproic acid • Depakote
Disclosures
Dr. Yule received grant support from the 2012 American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC, and receives grant support from the 2014 Louis V. Gerstner III Research Scholar Award. Dr. Wilens has received grant support from the National Institute on Drug Abuse (NIDA); has been a consultant for Euthymics/Neurovance, NIDA, Ironshore Pharmaceuticals and Development, Theravance Biopharma, Tris Pharma, the U.S. National Football League (ERM Associates), U.S. Minor/Major League Baseball, and Bay Cove Human Services (Clinical Services).
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.
1. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future, Table 2: trends in annual prevalence of use of various drugs in grades 8, 10, and 12. http://www. monitoringthefuture.org/data/14data.html#2014data-drugs. Published December 16, 2014. Accessed January 6, 2015.
2. Merikangas KR, He JP, Burnstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-- Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
3. Kandel DB, Johnson JG, Bird HR, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25(2):122-132.
4. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(suppl 1):S4-S13.
5. Stowell R, Estroff TW. Psychiatric disorders in substance-abusing adolescent inpatients: a pilot study. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1036-1040.
6. National Institute of Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: a practitioner’s guide. http://www.niaaa.nih.gov/ Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. Accessed March 11, 2015.
7. Children’s Hospital Boston. The CRAFFT screening interview. http://www.integration.samhsa.gov/clinical-practice/sbirt/CRAFFT_Screening_interview.pdf. Published 2009. Accessed March 11, 2015.
8. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Kelly JF, Myers MG. Adolescents’ participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259-269.
11. Lifrak PD, Alterman AI, O’Brien CP, et al. Naltrexone for alcoholic adolescents. Am J Psychiatry. 1997;154(3):439-441.
12. Deas D, May MP, Randall C, et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723-728.
13. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941-954.
14. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
15. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
16. Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23(3):231-237.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. (SMA) 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
18. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62(10):1157-1164.
19. Gowing L, Farrell MF, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:CD002024.
20. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008; 300(17):2003-2011.
21. Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669-1676.
22. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295-297.
23. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. J Subst Abuse Treat. 2008;13(6):382-388.
24. Deas D, Randall CL, Roberts JS, et al. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461-469.
25. Riggs PD, Mikulich-Gilbertson SK, Davies RD, et al. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026-1034.
26. Findling RL, Pagano ME, McNamara NK, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial. Child Adolesc Psychiatry Ment Health. 2009;3(1):11.
27. Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39-45.
28. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
29. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
30. Donovan SJ, Susser ES, Nunes E. Divalproex sodium for use with conduct disordered adolescent marijuana users. Am J Addict. 1996;5(2):181.
31. Donovan SJ, Susser ES, Nunes EV, et al. Divalproex treatment of disruptive adolescents: a report of 10 cases. J Clin Psychiatry. 1997;58(1):12-15.
32. DelBello, M. Topiramate plus quetiapine cut Cannabis use in bipolar teens. Paper presented at: American Academy of Child and Adolescent Psychiatry’s Annual Meeting. November 2011; Toronto, Ontario, Canada.
33. Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008;95(3):188-198.
34. Riggs PD, Leon SL, Mikulich SK, et al. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1271-1278.
35. Riggs PD, Hall SK, Mikulich-Gilbertson SK, et al. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(4):420-429.
36. Riggs PD, Winhusen T, Davies RD, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914.
37. Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate- SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41(3):250-257.
38. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005;15(5): 777-786.
39. Thurstone C, Riggs PD, Salomonsen-Sautel S, et al. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573-582.
40. Zulauf CA, Sprich SE, Safren SA, et al. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436.
41. Riggs PD, Mikulich SK, Coffman LM, et al. Fluoxetine in drug-dependent delinquents with major depression: an open trial. J Child Adolesc Psychopharmacol. 1997;7(2):87-95.
42. Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171(7):741-748.
43. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012; 21(3):527-539.
Lisdexamfetamine for ADHD
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
Attention-deficit/hyperactivity disorder: Tips to individualize drug therapy
Attention-deficit/hyperactivity disorder, or ADHD, affects 4% to 5% of youths worldwide and is the most common neurobehavioral disorder treated in children.1 Recent research and clinical experience are changing our understanding of ADHD in two important ways:
First, we now recognize that ADHD is often chronic. Its symptoms and/or associated impairment persist into adolescence in approximately three-quarters of cases and into adulthood in approximately one-half of childhood cases.2-3 Throughout the lifespan, ADHD is associated with significant psychopathology, school and occupational failure, and peer and emotional difficulties.4
Second, the presence of impaired cognition has largely replaced the view that ADHD was characterized primarily by overactivity and impulsivity.5 This insight is leading to innovations in pharmacotherapy that offer youths and adults improved control of ADHD symptoms, with less-frequent dosing and lower risk of side effects.
Neurobiology
Although the precise neurobiology of ADHD remains unknown, frontal network abnormality or frontal-striatal dysfunction appears critical.6 Catecholamine dysregulation affecting both the dopaminergic and noradrenergic systems appears to be important in the underlying pathophysiology.6 For example, a small replicated study using SPECT imaging found adults with ADHD had twice the dopamine transporter binding potential of age-matched controls.7 Recent data also suggest the cholinergic system is involved in mediating symptoms of ADHD, particularly attentional regulation. Data from adoption, twin, and family-genetic studies suggest a genetic contribution in ADHD, with molecular studies focusing on the dopamine D2, D4, and the dopamine transporter as candidate genes.8
Diagnosis
Symptoms of ADHD are related to the patient’s age at presentation. In youth, ADHD is characterized by inattention, distractibility, impulsivity, and hyperactivity excessive for the child’s developmental level.1,5 Other symptoms include low frustration tolerance, frequent shifting of activities, difficulty organizing tasks, and daydreaming. While these symptoms are typically pervasive, they may not occur in all settings.
Older adolescents and adults tend to present with prominent attentional difficulties (distractibility, shifting activities frequently, forgetfulness, disorganization) that affect work, schooling, and relationships.9 These older patients frequently also manifest residual impulsivity (intrusiveness, impatience) and hyperactivity (fidgetiness, restlessness).6 Adults with ADHD have a history of childhood onset of the disorder, with persistence through adolescence and beyond. Diagnosis of adult ADHD requires evidence of impairment in academic, work, and interpersonal domains.

- Either (1) or (2)
- Some hyperactive-impulsive or inattentive symptoms that caused impairment were present before age 7.
- Some impairment from the symptoms is present in two or more settings (e.g., at school/work or at home).
- There must be clear evidence of clinically significant impairment in social, academic, or occupational functioning.
- The symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychiatric disorder and are not better accounted for by a mood, anxiety, dissociative, personality, or other mental disorder.
Code based on type:
314.01 ADHD, Combined Type—if both criteria A1 and A2 have been met for the past 6 months.
314.00 ADHD, Predominantly Inattentive Type—if criterion A1 has been met but criterion A2 has not been met for the past 6 months.
314.01 ADHD, Predominantly Hyperactive-Impulsive Type—if criterion A2 has been met but criterion A1 has not been met for the past 6 months.
(Specify “In partial remission” in patients whose symptoms no longer meet full criteria).
Adapted from: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text revision. Washington: American Psychiatric Association, 2000.
DSM-IV recognizes three subtypes of ADHD based on presenting symptoms:
- predominantly inattentive (20% to 30% of cases);
- predominantly hyperactive-impulsive (<15%);
- combined inattentive and hyperactive-impulsive (50% to 75%).
ADHD is diagnosed by clinical history, applying DSM-IV criteria ( Table 1). Rating scales, checklists, and neuropsychological batteries—although not diagnostic—may help provide evidence for the disorder and accompanying comorbid conditions (e.g., Conners Rating Scales, Brown Rating Scales).5
Complicating the clinical picture of ADHD is the common co-occurrence of other psychiatric disorders. Almost three-quarters of individuals with ADHD have psychiatric comorbidity, including:
- oppositional disorders (40% to 60% of ADHD cases);
- conduct disorders (10% to 20%);
- anxiety disorders (30% to 40%);
- mood disorders (20% to 30%).10
For example, although few people with ADHD develop bipolar illness, an excess of ADHD is reported in depressed (20% to 30%) and bipolar youth (50% to 90%).11 ADHD and its associated comorbid conditions also place sufferers at risk for higher rates and younger onset of cigarette smoking and substance abuse.12 Most studies, however, indicate that pharmacotherapy reduces the risk for later drug and alcohol use disorders.13
Treatment
Management of ADHD includes nonpharmacologic and pharmacologic interventions.1 Support groups (e.g., Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD), www.chadd.org) are invaluable and inexpensive sources of information about ADHD.
For children in school, a specialized educational plan with frequent re-evaluations of the child’s progress is recommended. Encourage parents to work closely with the child’s teacher, guidance counselor, or school psychologist. Children with ADHD tend to perform better in school when given structure, a predictable routine, checked homework, learning aids, and resource room time.5 Specific remediation plans are recommended for comorbid learning disorders, found in approximately one-third of individuals with ADHD.
Adults with ADHD may need to modify their school or work settings to function well. College students should be encouraged to use their school’s study center, and may require accommodations for taking examinations.
Focused cognitive behavioral therapies have shown benefit in children, adolescents, and adults with ADHD.14 Training children and their parents in behavioral modification can help control the child’s disruptive behaviors, inflexibility, anxiety, or outbursts. Other useful adjuncts to treatment include remediation to improve interpersonal skills and coaching to address organization and study skills.
Pharmacotherapy
Medications are fundamental in treating ADHD1 (Table 2). In fact, a 14-month, multisite study demonstrated that medication management of ADHD was the most important variable in outcome when patients received combined pharmacologic and nonpharmacologic therapies.15 Stimulants, antihypertensives, and antidepressants are used to treat ADHD symptoms. Children, adolescents, and adults with ADHD respond similarly to pharmacotherapy.16
Psychostimulants: First-line agents
Psychostimulants are first-line agents for ADHD, based in part on extensive data showing efficacy (>250 controlled trials) and safety.17,18 Stimulants are sympathomimetic drugs that increase intrasynaptic catecholamines (mainly dopamine) by inhibiting the presynaptic reuptake mechanism (amphetamine, methylphenidate, and pemoline) and releasing presynaptic catecholamines (amphetamine).19 Methylphenidate, dextroamphetamine, amphetamine compounds, and magnesium pemoline are among the most commonly used compounds in this class.
New approaches Prescribing stimulants for ADHD has changed in two fundamental ways. Frist, in the past we covered a child’s ADHD symptoms only during school hours, but we now include time after school and weekends and holidays. Second, we also are using longer-acting stimulant preparations, which recently became available. Extended-release preparations are usually preferred for lack of in-school dosing requirements, improved compliance, reduced stigma and wear-off, and lower risk of abuse or diversion—i.e., the medication being given or sold by an individual with ADHD to someone who is using it recreationally.
Short-acting compounds such as methylphenidate, D-methylphenidate, and D-amphetamine begin working within 30 to 60 minutes. Their clinical effect usually peaks 1 and 2 hours after administration and lasts 2 to 5 hours. The amphetamine compounds (e.g., Adderall) and older sustained-release methylphenidate begin working within 60 minutes, with a clinical effect that usually peaks between 1 and 3 hours and is maintained for 5 to 8 hours).
Table 2
RECOMMENDED DOSING OF PSYCHOSTIMULANTS FOR ADHD
| Medication | Starting dosage | Maximum dosage | Usual dosing (hr) |
|---|---|---|---|
| Methylphenidate (short-acting) | |||
| Ritalin | 5 mg bid | 2 mg/kg/day | tid (4 hr) |
| Dexmethylphenidate (short-acting) | |||
| Focalin | 2.5 mg bid | 1 mg/kg/day | bid (5 hr) |
| Methylphenidate (extended-release) | |||
| Concerta | 18 mg once daily | 2 mg/kg/day | Once (12 hr) |
| Metadate CD | 20 mg once daily | Once (8-9 hr) | |
| Ritalin LA | 10 mg once daily | Once (8-9 hr) | |
| Amphetamine compounds | |||
| Adderall | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid (6 hr) |
| Adderall XR | 10 mg | Once (12 hr) | |
| Dextroamphetamine | |||
| Dexedrine | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid/tid (4 hr) |
| Dex Spansule | 5 mg | bid (6 hr) | |
| Magnesium pemoline | |||
| Cylert | 37.5 mg once in the morning | 3 mg/kg/day | Once |
Newer extended-release methylphenidate products (e.g., Ritalin LA and Metadate CD), with 8 to 9 hours’ duration of action, were developed to approximate twice-daily short-acting methylphenidate. The Concerta brand of methylphenidate, with 10 to 12 hours’ duration of action, approximates short-acting methylphenidate given three times daily. The extended-release Adderall XR brand of amphetamine compound, with a 10- to 12-hour duration of action, is similar to twice-daily Adderall.
Methylphenidate is the most studied, but among the available stimulants the literature suggests more similarities than differences in patient response.17,18 Because of the agents’ marginally different mechanisms of action, however, some patients who do not respond satisfactorily to one stimulant or manifest adverse effects may respond more favorably to another agent of this type.
Start stimulants at the lowest available dose and increase every 3 to 4 days until a response is noted or adverse effects emerge. Dose-response data indicate more robust response at higher dosages of stimulants; therefore, efficacy—rather than onset of side effects—should guide titration to an optimal dose.
Predictable short-term adverse effects include reduced appetite, insomnia, edginess, and GI upset.20 To manage these effects, consider when they occur:
- Within 2 hours after administration may signal the need to reduce the dose or change to another preparation.
- Within 4 to 6 hours after administration (e.g., moodiness) suggests the need for a longer-acting preparation or low dosing prior to the anticipated wear-off.
For insomnia, strategies include using a shorter-acting stimulant preparation, reducing the stimulant load in the afternoon, or providing adjunct treatment for the insomnia (i.e., clonidine, imipramine, mirtazapine).17 Edginess and headaches—more common in adolescents and adults—can be reduced with low-dose beta blockers. For diminished appetite in youths, caloric intake can be enhanced with a hearty breakfast, late-afternoon and evening snacks, and caloric supplements. Appetite enhancers such as cyproheptadine given nightly may be considered. Pemoline may rarely cause hepatitis and requires liver function monitoring.
Chronic use of stimulants is controversial.17,18 Although stimulants may produce anorexia and weight loss, their effect on a youth’s ultimate height is less certain. Initial reports of a persistent stimulant-associated growth decrease have not been substantiated. Other studies suggest that growth deficits may represent maturational delays related to ADHD rather than to stimulant treatment.21
Stimulants may precipitate or exacerbate tic symptoms in children with ADHD. Recent work suggests that stimulants can be used safely in youth with tic disorders,22 although up to one-third may experience worsening of tic symptoms.
Despite case reports of stimulant misuse, there is little data to support stimulant abuse among treated children with ADHD.13 However, the diversion of stimulants to youth without ADHD is a concern.
Antidepressants
Antidepressants are generally considered second-line drugs for ADHD.1,16 Bupropion, an antidepressant with indirect dopamine and noradrenergic effects, has been shown effective in ADHDin controlled trials of both children and adults.23,24
Bupropion is often prescribed first for complex patients with ADHD and substance abuse or an unstable mood disorder because of its ability to reduce cigarette smoking and improve mood, lack of monitoring requirements, and few adverse effects. Dosing is typically initiated at 100 mg of the sustained-release preparation and increased weekly to a maximum of 300 mg in younger children and 400 mg in older children or adults (i.e., 200 mg bid). Adverse effects include insomnia, activation, irritability, and (rarely) seizures.
The tricyclic antidepressants (TCAs) used in ADHD—imipramine, desipramine and nortriptyline—block the reuptake of neurotransmitters including norepinephrine. TCAs are effective in controlling abnormal behaviors and improving cognitive impairments associated with ADHD, but less so than the stimulants. TCAs are particularly useful when:
- stimulants fail to control ADHD symptoms;
- oppositional behavior, anxiety, tics, or depressive symptoms coexist within ADHD or occur during its treatment.
Desipramine appears to be the most effective TCA for ADHD, followed by nortriptyline and imipramine.25,26 TCAs are dosed starting with 25 mg/d and slowly increased to a maximum of 5 mg/kg/day (2 mg/kg/day for nortriptyline). Although immediate relief can be seen, a delay of up to 6 weeks for maximal effect is common. Typical adverse effects include dry mouth, constipation, sedation, and weight gain.
Four deaths have been reported in children with ADHD treated with desipramine; however, independent evaluation of these cases failed to support a causal link. As minor increases in heart rate and ECG intervals are predictable with TCAs, ECG monitoring at baseline and at therapeutic dosages is recommended.
Although serotonin reuptake inhibitors are not generally useful for ADHD, venlafaxine appears to have mild efficacy, perhaps because of its dose-dependent noradrenergic reuptake inhibition.27
Monoamine oxidase inhibitors (MAOIs) have been shown effective in juvenile ADHD. Response to treatment is rapid, and standard antidepressant dosing is often necessary.16 A major limitation to the use of MAOIs is the potential for hypertensive crisis associated with dietetic transgressions and drug interactions.
Other treatment options
Antihypertensives The antihypertensive agents clonidine28 and guanfacine29 are used to treat the hyperactive-impulsive symptoms of ADHD in youth. Clonidine is relatively shortacting, with usual daily dosage ranges from 0.05 to 0.4 mg.28 Guanfacine is longer acting and less potent, with usual daily dosage ranges from 0.5 to 4 mg.29
Antihypertensives have been used to treat ADHD and associated tics, aggression, and sleep disturbances, particularly in younger children.16 Although sedation is more common with clonidine than guanfacine, both agents may cause depression and rebound hypertension. Cardiovascular monitoring (vital signs, ECG) remains optional.
New agents Novel compounds, along with new preparations and delivery systems of existing stimulant medications, are being investigated for managing ADHD. New agents are being tested in adults with ADHD because adults and youth respond similarly to ADHD medications, and there are ethical concerns about drug testing in children.
Atomoxetine, a noradrenergic reuptake inhibitor under development, has been shown in open and controlled studies of adults and youth30 to be effective in treating ADHD. Atomoxetine appears well tolerated, with no blood monitoring requirements.
Cholinergics and genes Selective use of cholinergic agents (e.g., donepezil) may also be helpful for the cognitive dysfunction in ADHD,24 either as monotherapy or in combination with other agents for ADHD. Multiple centers are investigating the possible link between response to pharmacologic therapy and ADHD genotype.
Combination therapy
Combinations of pharmacologic agents can be used to treat comorbid ADHD, to augment response to a single agent, for pharmacokinetic synergism, and to manage adverse effects that emerge during treatment. Examples include:
- a tricyclic antidepressant and a stimulant to heighten response to treatment;
- an antidepressant plus a stimulant for ADHD and comorbid depression;
- adjunctive use of clonidine for sleep or to manage aggressive behavior;
- use of mood stabilizers with ADHD medications for comorbid bipolar disorder.16
Pharmacologic intervention for prominent concomitant mood disorders (depression and bipolarity) and anxiety should be sequenced prior to ADHD treatment.
Summary of treatment recommendations
Based on efficacy and safety, stimulants are first-line agents for routine management of ADHD, followed by antidepressants and antihypertensives. Patients who do not respond to the initial stimulant or who manifest adverse effects should be considered for a trial with an alternate stimulant. If two stimulant trials are unsuccessful, bupropion and the tricyclic antidepressants are reasonable second-line agents.
Antihypertensives alone or in combination with other ADHD medication may help youths with tics,31 prominent hyperactivity, impulsivity, or aggressiveness. MAOIs may be considered for refractory patients, and cholinergic agents (e.g., donepezil) may be used for excessive cognitive difficulties such as organization, planning, and time management.
Related resources
- Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: The Guilford Press, 1998.
- Wilens T. Straight Talk About Psychiatric Medications for Kids. New York: The Guilford Press, 1998.
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD), www.chadd.org
Drug brand names
- Atomoxetine • (under development)
- Bupropion • Wellbutrin
- Clonidine • Catapress
- Dextro-amphetamine • Dexedrine
- Dexmethylphenidate • Focalin
- Donepezil • Aricept
- Guanfacine • Tenex
- Methylphenidate • Ritalin, Concerta, Metadate
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
Dr. Biederman reports that he receives research/grant support from, and is on the speaker’s bureau and advisory boards of Eli Lilly & Co. and Shire Laboratories. He also reports that he receives research/grant support from Wyeth-Ayerst Pharmaceuticals, Pfizer Inc., Cephalon Pharmaceutical, Janssen Pharmaceutica, and Noven Pharmaceutical; is on the speaker's bureau of GlaxoSmithKline, Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Alza/McNeil Pharmaceutical and Cephalon Pharmaceutical; and is on the advisory board of Cell Tech, Noven Pharmaceutical, and Alza/McNeil Pharmaceuticals.
Drs. Wilens and Spencer report that they receive research/grant support from, are on the speakers bureau of, and/or serve as consultants to Abbott Laboratories, McNeil Pharmaceuticals, Celltech Medieva, GlaxoSmithKline, Eli Lilly & Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth-Ayerst Pharmaceuticals.
1. Goldman L, Genel M, Bezman R, Slanetz P. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA 1998;279:1100-7.
2. Hechtman L, Weiss G. Controlled prospective fifteen-year follow-up of hyperactives as adults: mon-medical drug and alcohol use and anti-social behaviour. Can J Psychiatry 1986;31:557-67.
3. Fischer M. Persistence of ADHD into adulthood: it depends on whom you ask. The ADHD Report 1997;5:8-10.
4. Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 1998;59:4-16.
5. Barkley R. Attention-deficit/hyperactivity disorder: A handbook for diagnosis and treament (2nd ed). New York: Guilford Press, 1998.
6. Zametkin A, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 1998;59:17-23.
7. Dougherty D, Bonab A, Spencer T, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999;354:2132-3.
8. Faraone SV, Biederman J, Weiffenbach B, et al. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:768-70.
9. Millstein RB, Wilens TE, Biederman J, Spencer TJ. Presenting ADHD symptoms and subtypes in clincially referred adults with ADHD. J Attent Disord 1997;2:159-66.
10. Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 1991;148:564-77.
11. Woznia J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis 1997;185:475-82.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone S. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
14. Abikoff H. Cognitive training in ADHD children; less to It than meets the eye. J Learn Disabil 1991;24:205-9.
15. Group MTS. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-86.
16. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention deficit disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 1996;35:409-32.
17. Wilens T, Spencer T. The stimulants revisited. In: Stubbe C. Child an adolescent psychiatric clinics of North America. Philadelphia: JB Saunders, 2000;573-603
18. Greenhill L, Osman B. Ritalin: theory and practice. New York: Mary Ann Liebert, 1999.
19. Elia J, Borcherding BG, Potter WZ, et al. Stimulant drug treatment of hyperactivity: biochemical correlates. Clin Pharmacol Ther 1990;48:57-66.
20. Barkley RA, McMurray MB, Edelbrock CS, Robbin K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86:184-92.
21. Spencer TJ, Biederman J, Harding M, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry 1996;35:1460-9.
22. Gadow K, Sverd J, Sprafkin J, Nolan E, Grossman S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56:330-6.
23. Conners CK, Casat CD, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 1996;35:1314-21.
24. Wilens T, Biederman J, Spencer T, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:1931-7.
25. Biederman J, Baldessarini RJ, Wright V, Knee D, Harmatz JS. A double-blind placebo controlled study of desipramine in the treatment of ADD. I. Efficacy. J Am Acad Child Adolesc Psychiatry 1989;28:777-784.
26. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2000;10:193-204.
27. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/ hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57:184-9.
28. Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Adolesc Psychiatry 1985;24:617-29.
29. Horrigan JP, Barnhill LJ. Guanfacine for treatment of attention-deficit hyperactivity disorder in boys. J Child Adolesc Psychopharmacol 1995;5:215-23.
30. Kratochvil CJ, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2001;11:167-70.
31. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics. A ramdomized controlled trial. Neurology 2002;58(4):527-36.
Attention-deficit/hyperactivity disorder, or ADHD, affects 4% to 5% of youths worldwide and is the most common neurobehavioral disorder treated in children.1 Recent research and clinical experience are changing our understanding of ADHD in two important ways:
First, we now recognize that ADHD is often chronic. Its symptoms and/or associated impairment persist into adolescence in approximately three-quarters of cases and into adulthood in approximately one-half of childhood cases.2-3 Throughout the lifespan, ADHD is associated with significant psychopathology, school and occupational failure, and peer and emotional difficulties.4
Second, the presence of impaired cognition has largely replaced the view that ADHD was characterized primarily by overactivity and impulsivity.5 This insight is leading to innovations in pharmacotherapy that offer youths and adults improved control of ADHD symptoms, with less-frequent dosing and lower risk of side effects.
Neurobiology
Although the precise neurobiology of ADHD remains unknown, frontal network abnormality or frontal-striatal dysfunction appears critical.6 Catecholamine dysregulation affecting both the dopaminergic and noradrenergic systems appears to be important in the underlying pathophysiology.6 For example, a small replicated study using SPECT imaging found adults with ADHD had twice the dopamine transporter binding potential of age-matched controls.7 Recent data also suggest the cholinergic system is involved in mediating symptoms of ADHD, particularly attentional regulation. Data from adoption, twin, and family-genetic studies suggest a genetic contribution in ADHD, with molecular studies focusing on the dopamine D2, D4, and the dopamine transporter as candidate genes.8
Diagnosis
Symptoms of ADHD are related to the patient’s age at presentation. In youth, ADHD is characterized by inattention, distractibility, impulsivity, and hyperactivity excessive for the child’s developmental level.1,5 Other symptoms include low frustration tolerance, frequent shifting of activities, difficulty organizing tasks, and daydreaming. While these symptoms are typically pervasive, they may not occur in all settings.
Older adolescents and adults tend to present with prominent attentional difficulties (distractibility, shifting activities frequently, forgetfulness, disorganization) that affect work, schooling, and relationships.9 These older patients frequently also manifest residual impulsivity (intrusiveness, impatience) and hyperactivity (fidgetiness, restlessness).6 Adults with ADHD have a history of childhood onset of the disorder, with persistence through adolescence and beyond. Diagnosis of adult ADHD requires evidence of impairment in academic, work, and interpersonal domains.

- Either (1) or (2)
- Some hyperactive-impulsive or inattentive symptoms that caused impairment were present before age 7.
- Some impairment from the symptoms is present in two or more settings (e.g., at school/work or at home).
- There must be clear evidence of clinically significant impairment in social, academic, or occupational functioning.
- The symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychiatric disorder and are not better accounted for by a mood, anxiety, dissociative, personality, or other mental disorder.
Code based on type:
314.01 ADHD, Combined Type—if both criteria A1 and A2 have been met for the past 6 months.
314.00 ADHD, Predominantly Inattentive Type—if criterion A1 has been met but criterion A2 has not been met for the past 6 months.
314.01 ADHD, Predominantly Hyperactive-Impulsive Type—if criterion A2 has been met but criterion A1 has not been met for the past 6 months.
(Specify “In partial remission” in patients whose symptoms no longer meet full criteria).
Adapted from: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text revision. Washington: American Psychiatric Association, 2000.
DSM-IV recognizes three subtypes of ADHD based on presenting symptoms:
- predominantly inattentive (20% to 30% of cases);
- predominantly hyperactive-impulsive (<15%);
- combined inattentive and hyperactive-impulsive (50% to 75%).
ADHD is diagnosed by clinical history, applying DSM-IV criteria ( Table 1). Rating scales, checklists, and neuropsychological batteries—although not diagnostic—may help provide evidence for the disorder and accompanying comorbid conditions (e.g., Conners Rating Scales, Brown Rating Scales).5
Complicating the clinical picture of ADHD is the common co-occurrence of other psychiatric disorders. Almost three-quarters of individuals with ADHD have psychiatric comorbidity, including:
- oppositional disorders (40% to 60% of ADHD cases);
- conduct disorders (10% to 20%);
- anxiety disorders (30% to 40%);
- mood disorders (20% to 30%).10
For example, although few people with ADHD develop bipolar illness, an excess of ADHD is reported in depressed (20% to 30%) and bipolar youth (50% to 90%).11 ADHD and its associated comorbid conditions also place sufferers at risk for higher rates and younger onset of cigarette smoking and substance abuse.12 Most studies, however, indicate that pharmacotherapy reduces the risk for later drug and alcohol use disorders.13
Treatment
Management of ADHD includes nonpharmacologic and pharmacologic interventions.1 Support groups (e.g., Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD), www.chadd.org) are invaluable and inexpensive sources of information about ADHD.
For children in school, a specialized educational plan with frequent re-evaluations of the child’s progress is recommended. Encourage parents to work closely with the child’s teacher, guidance counselor, or school psychologist. Children with ADHD tend to perform better in school when given structure, a predictable routine, checked homework, learning aids, and resource room time.5 Specific remediation plans are recommended for comorbid learning disorders, found in approximately one-third of individuals with ADHD.
Adults with ADHD may need to modify their school or work settings to function well. College students should be encouraged to use their school’s study center, and may require accommodations for taking examinations.
Focused cognitive behavioral therapies have shown benefit in children, adolescents, and adults with ADHD.14 Training children and their parents in behavioral modification can help control the child’s disruptive behaviors, inflexibility, anxiety, or outbursts. Other useful adjuncts to treatment include remediation to improve interpersonal skills and coaching to address organization and study skills.
Pharmacotherapy
Medications are fundamental in treating ADHD1 (Table 2). In fact, a 14-month, multisite study demonstrated that medication management of ADHD was the most important variable in outcome when patients received combined pharmacologic and nonpharmacologic therapies.15 Stimulants, antihypertensives, and antidepressants are used to treat ADHD symptoms. Children, adolescents, and adults with ADHD respond similarly to pharmacotherapy.16
Psychostimulants: First-line agents
Psychostimulants are first-line agents for ADHD, based in part on extensive data showing efficacy (>250 controlled trials) and safety.17,18 Stimulants are sympathomimetic drugs that increase intrasynaptic catecholamines (mainly dopamine) by inhibiting the presynaptic reuptake mechanism (amphetamine, methylphenidate, and pemoline) and releasing presynaptic catecholamines (amphetamine).19 Methylphenidate, dextroamphetamine, amphetamine compounds, and magnesium pemoline are among the most commonly used compounds in this class.
New approaches Prescribing stimulants for ADHD has changed in two fundamental ways. Frist, in the past we covered a child’s ADHD symptoms only during school hours, but we now include time after school and weekends and holidays. Second, we also are using longer-acting stimulant preparations, which recently became available. Extended-release preparations are usually preferred for lack of in-school dosing requirements, improved compliance, reduced stigma and wear-off, and lower risk of abuse or diversion—i.e., the medication being given or sold by an individual with ADHD to someone who is using it recreationally.
Short-acting compounds such as methylphenidate, D-methylphenidate, and D-amphetamine begin working within 30 to 60 minutes. Their clinical effect usually peaks 1 and 2 hours after administration and lasts 2 to 5 hours. The amphetamine compounds (e.g., Adderall) and older sustained-release methylphenidate begin working within 60 minutes, with a clinical effect that usually peaks between 1 and 3 hours and is maintained for 5 to 8 hours).
Table 2
RECOMMENDED DOSING OF PSYCHOSTIMULANTS FOR ADHD
| Medication | Starting dosage | Maximum dosage | Usual dosing (hr) |
|---|---|---|---|
| Methylphenidate (short-acting) | |||
| Ritalin | 5 mg bid | 2 mg/kg/day | tid (4 hr) |
| Dexmethylphenidate (short-acting) | |||
| Focalin | 2.5 mg bid | 1 mg/kg/day | bid (5 hr) |
| Methylphenidate (extended-release) | |||
| Concerta | 18 mg once daily | 2 mg/kg/day | Once (12 hr) |
| Metadate CD | 20 mg once daily | Once (8-9 hr) | |
| Ritalin LA | 10 mg once daily | Once (8-9 hr) | |
| Amphetamine compounds | |||
| Adderall | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid (6 hr) |
| Adderall XR | 10 mg | Once (12 hr) | |
| Dextroamphetamine | |||
| Dexedrine | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid/tid (4 hr) |
| Dex Spansule | 5 mg | bid (6 hr) | |
| Magnesium pemoline | |||
| Cylert | 37.5 mg once in the morning | 3 mg/kg/day | Once |
Newer extended-release methylphenidate products (e.g., Ritalin LA and Metadate CD), with 8 to 9 hours’ duration of action, were developed to approximate twice-daily short-acting methylphenidate. The Concerta brand of methylphenidate, with 10 to 12 hours’ duration of action, approximates short-acting methylphenidate given three times daily. The extended-release Adderall XR brand of amphetamine compound, with a 10- to 12-hour duration of action, is similar to twice-daily Adderall.
Methylphenidate is the most studied, but among the available stimulants the literature suggests more similarities than differences in patient response.17,18 Because of the agents’ marginally different mechanisms of action, however, some patients who do not respond satisfactorily to one stimulant or manifest adverse effects may respond more favorably to another agent of this type.
Start stimulants at the lowest available dose and increase every 3 to 4 days until a response is noted or adverse effects emerge. Dose-response data indicate more robust response at higher dosages of stimulants; therefore, efficacy—rather than onset of side effects—should guide titration to an optimal dose.
Predictable short-term adverse effects include reduced appetite, insomnia, edginess, and GI upset.20 To manage these effects, consider when they occur:
- Within 2 hours after administration may signal the need to reduce the dose or change to another preparation.
- Within 4 to 6 hours after administration (e.g., moodiness) suggests the need for a longer-acting preparation or low dosing prior to the anticipated wear-off.
For insomnia, strategies include using a shorter-acting stimulant preparation, reducing the stimulant load in the afternoon, or providing adjunct treatment for the insomnia (i.e., clonidine, imipramine, mirtazapine).17 Edginess and headaches—more common in adolescents and adults—can be reduced with low-dose beta blockers. For diminished appetite in youths, caloric intake can be enhanced with a hearty breakfast, late-afternoon and evening snacks, and caloric supplements. Appetite enhancers such as cyproheptadine given nightly may be considered. Pemoline may rarely cause hepatitis and requires liver function monitoring.
Chronic use of stimulants is controversial.17,18 Although stimulants may produce anorexia and weight loss, their effect on a youth’s ultimate height is less certain. Initial reports of a persistent stimulant-associated growth decrease have not been substantiated. Other studies suggest that growth deficits may represent maturational delays related to ADHD rather than to stimulant treatment.21
Stimulants may precipitate or exacerbate tic symptoms in children with ADHD. Recent work suggests that stimulants can be used safely in youth with tic disorders,22 although up to one-third may experience worsening of tic symptoms.
Despite case reports of stimulant misuse, there is little data to support stimulant abuse among treated children with ADHD.13 However, the diversion of stimulants to youth without ADHD is a concern.
Antidepressants
Antidepressants are generally considered second-line drugs for ADHD.1,16 Bupropion, an antidepressant with indirect dopamine and noradrenergic effects, has been shown effective in ADHDin controlled trials of both children and adults.23,24
Bupropion is often prescribed first for complex patients with ADHD and substance abuse or an unstable mood disorder because of its ability to reduce cigarette smoking and improve mood, lack of monitoring requirements, and few adverse effects. Dosing is typically initiated at 100 mg of the sustained-release preparation and increased weekly to a maximum of 300 mg in younger children and 400 mg in older children or adults (i.e., 200 mg bid). Adverse effects include insomnia, activation, irritability, and (rarely) seizures.
The tricyclic antidepressants (TCAs) used in ADHD—imipramine, desipramine and nortriptyline—block the reuptake of neurotransmitters including norepinephrine. TCAs are effective in controlling abnormal behaviors and improving cognitive impairments associated with ADHD, but less so than the stimulants. TCAs are particularly useful when:
- stimulants fail to control ADHD symptoms;
- oppositional behavior, anxiety, tics, or depressive symptoms coexist within ADHD or occur during its treatment.
Desipramine appears to be the most effective TCA for ADHD, followed by nortriptyline and imipramine.25,26 TCAs are dosed starting with 25 mg/d and slowly increased to a maximum of 5 mg/kg/day (2 mg/kg/day for nortriptyline). Although immediate relief can be seen, a delay of up to 6 weeks for maximal effect is common. Typical adverse effects include dry mouth, constipation, sedation, and weight gain.
Four deaths have been reported in children with ADHD treated with desipramine; however, independent evaluation of these cases failed to support a causal link. As minor increases in heart rate and ECG intervals are predictable with TCAs, ECG monitoring at baseline and at therapeutic dosages is recommended.
Although serotonin reuptake inhibitors are not generally useful for ADHD, venlafaxine appears to have mild efficacy, perhaps because of its dose-dependent noradrenergic reuptake inhibition.27
Monoamine oxidase inhibitors (MAOIs) have been shown effective in juvenile ADHD. Response to treatment is rapid, and standard antidepressant dosing is often necessary.16 A major limitation to the use of MAOIs is the potential for hypertensive crisis associated with dietetic transgressions and drug interactions.
Other treatment options
Antihypertensives The antihypertensive agents clonidine28 and guanfacine29 are used to treat the hyperactive-impulsive symptoms of ADHD in youth. Clonidine is relatively shortacting, with usual daily dosage ranges from 0.05 to 0.4 mg.28 Guanfacine is longer acting and less potent, with usual daily dosage ranges from 0.5 to 4 mg.29
Antihypertensives have been used to treat ADHD and associated tics, aggression, and sleep disturbances, particularly in younger children.16 Although sedation is more common with clonidine than guanfacine, both agents may cause depression and rebound hypertension. Cardiovascular monitoring (vital signs, ECG) remains optional.
New agents Novel compounds, along with new preparations and delivery systems of existing stimulant medications, are being investigated for managing ADHD. New agents are being tested in adults with ADHD because adults and youth respond similarly to ADHD medications, and there are ethical concerns about drug testing in children.
Atomoxetine, a noradrenergic reuptake inhibitor under development, has been shown in open and controlled studies of adults and youth30 to be effective in treating ADHD. Atomoxetine appears well tolerated, with no blood monitoring requirements.
Cholinergics and genes Selective use of cholinergic agents (e.g., donepezil) may also be helpful for the cognitive dysfunction in ADHD,24 either as monotherapy or in combination with other agents for ADHD. Multiple centers are investigating the possible link between response to pharmacologic therapy and ADHD genotype.
Combination therapy
Combinations of pharmacologic agents can be used to treat comorbid ADHD, to augment response to a single agent, for pharmacokinetic synergism, and to manage adverse effects that emerge during treatment. Examples include:
- a tricyclic antidepressant and a stimulant to heighten response to treatment;
- an antidepressant plus a stimulant for ADHD and comorbid depression;
- adjunctive use of clonidine for sleep or to manage aggressive behavior;
- use of mood stabilizers with ADHD medications for comorbid bipolar disorder.16
Pharmacologic intervention for prominent concomitant mood disorders (depression and bipolarity) and anxiety should be sequenced prior to ADHD treatment.
Summary of treatment recommendations
Based on efficacy and safety, stimulants are first-line agents for routine management of ADHD, followed by antidepressants and antihypertensives. Patients who do not respond to the initial stimulant or who manifest adverse effects should be considered for a trial with an alternate stimulant. If two stimulant trials are unsuccessful, bupropion and the tricyclic antidepressants are reasonable second-line agents.
Antihypertensives alone or in combination with other ADHD medication may help youths with tics,31 prominent hyperactivity, impulsivity, or aggressiveness. MAOIs may be considered for refractory patients, and cholinergic agents (e.g., donepezil) may be used for excessive cognitive difficulties such as organization, planning, and time management.
Related resources
- Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: The Guilford Press, 1998.
- Wilens T. Straight Talk About Psychiatric Medications for Kids. New York: The Guilford Press, 1998.
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD), www.chadd.org
Drug brand names
- Atomoxetine • (under development)
- Bupropion • Wellbutrin
- Clonidine • Catapress
- Dextro-amphetamine • Dexedrine
- Dexmethylphenidate • Focalin
- Donepezil • Aricept
- Guanfacine • Tenex
- Methylphenidate • Ritalin, Concerta, Metadate
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
Dr. Biederman reports that he receives research/grant support from, and is on the speaker’s bureau and advisory boards of Eli Lilly & Co. and Shire Laboratories. He also reports that he receives research/grant support from Wyeth-Ayerst Pharmaceuticals, Pfizer Inc., Cephalon Pharmaceutical, Janssen Pharmaceutica, and Noven Pharmaceutical; is on the speaker's bureau of GlaxoSmithKline, Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Alza/McNeil Pharmaceutical and Cephalon Pharmaceutical; and is on the advisory board of Cell Tech, Noven Pharmaceutical, and Alza/McNeil Pharmaceuticals.
Drs. Wilens and Spencer report that they receive research/grant support from, are on the speakers bureau of, and/or serve as consultants to Abbott Laboratories, McNeil Pharmaceuticals, Celltech Medieva, GlaxoSmithKline, Eli Lilly & Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth-Ayerst Pharmaceuticals.
Attention-deficit/hyperactivity disorder, or ADHD, affects 4% to 5% of youths worldwide and is the most common neurobehavioral disorder treated in children.1 Recent research and clinical experience are changing our understanding of ADHD in two important ways:
First, we now recognize that ADHD is often chronic. Its symptoms and/or associated impairment persist into adolescence in approximately three-quarters of cases and into adulthood in approximately one-half of childhood cases.2-3 Throughout the lifespan, ADHD is associated with significant psychopathology, school and occupational failure, and peer and emotional difficulties.4
Second, the presence of impaired cognition has largely replaced the view that ADHD was characterized primarily by overactivity and impulsivity.5 This insight is leading to innovations in pharmacotherapy that offer youths and adults improved control of ADHD symptoms, with less-frequent dosing and lower risk of side effects.
Neurobiology
Although the precise neurobiology of ADHD remains unknown, frontal network abnormality or frontal-striatal dysfunction appears critical.6 Catecholamine dysregulation affecting both the dopaminergic and noradrenergic systems appears to be important in the underlying pathophysiology.6 For example, a small replicated study using SPECT imaging found adults with ADHD had twice the dopamine transporter binding potential of age-matched controls.7 Recent data also suggest the cholinergic system is involved in mediating symptoms of ADHD, particularly attentional regulation. Data from adoption, twin, and family-genetic studies suggest a genetic contribution in ADHD, with molecular studies focusing on the dopamine D2, D4, and the dopamine transporter as candidate genes.8
Diagnosis
Symptoms of ADHD are related to the patient’s age at presentation. In youth, ADHD is characterized by inattention, distractibility, impulsivity, and hyperactivity excessive for the child’s developmental level.1,5 Other symptoms include low frustration tolerance, frequent shifting of activities, difficulty organizing tasks, and daydreaming. While these symptoms are typically pervasive, they may not occur in all settings.
Older adolescents and adults tend to present with prominent attentional difficulties (distractibility, shifting activities frequently, forgetfulness, disorganization) that affect work, schooling, and relationships.9 These older patients frequently also manifest residual impulsivity (intrusiveness, impatience) and hyperactivity (fidgetiness, restlessness).6 Adults with ADHD have a history of childhood onset of the disorder, with persistence through adolescence and beyond. Diagnosis of adult ADHD requires evidence of impairment in academic, work, and interpersonal domains.

- Either (1) or (2)
- Some hyperactive-impulsive or inattentive symptoms that caused impairment were present before age 7.
- Some impairment from the symptoms is present in two or more settings (e.g., at school/work or at home).
- There must be clear evidence of clinically significant impairment in social, academic, or occupational functioning.
- The symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychiatric disorder and are not better accounted for by a mood, anxiety, dissociative, personality, or other mental disorder.
Code based on type:
314.01 ADHD, Combined Type—if both criteria A1 and A2 have been met for the past 6 months.
314.00 ADHD, Predominantly Inattentive Type—if criterion A1 has been met but criterion A2 has not been met for the past 6 months.
314.01 ADHD, Predominantly Hyperactive-Impulsive Type—if criterion A2 has been met but criterion A1 has not been met for the past 6 months.
(Specify “In partial remission” in patients whose symptoms no longer meet full criteria).
Adapted from: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text revision. Washington: American Psychiatric Association, 2000.
DSM-IV recognizes three subtypes of ADHD based on presenting symptoms:
- predominantly inattentive (20% to 30% of cases);
- predominantly hyperactive-impulsive (<15%);
- combined inattentive and hyperactive-impulsive (50% to 75%).
ADHD is diagnosed by clinical history, applying DSM-IV criteria ( Table 1). Rating scales, checklists, and neuropsychological batteries—although not diagnostic—may help provide evidence for the disorder and accompanying comorbid conditions (e.g., Conners Rating Scales, Brown Rating Scales).5
Complicating the clinical picture of ADHD is the common co-occurrence of other psychiatric disorders. Almost three-quarters of individuals with ADHD have psychiatric comorbidity, including:
- oppositional disorders (40% to 60% of ADHD cases);
- conduct disorders (10% to 20%);
- anxiety disorders (30% to 40%);
- mood disorders (20% to 30%).10
For example, although few people with ADHD develop bipolar illness, an excess of ADHD is reported in depressed (20% to 30%) and bipolar youth (50% to 90%).11 ADHD and its associated comorbid conditions also place sufferers at risk for higher rates and younger onset of cigarette smoking and substance abuse.12 Most studies, however, indicate that pharmacotherapy reduces the risk for later drug and alcohol use disorders.13
Treatment
Management of ADHD includes nonpharmacologic and pharmacologic interventions.1 Support groups (e.g., Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD), www.chadd.org) are invaluable and inexpensive sources of information about ADHD.
For children in school, a specialized educational plan with frequent re-evaluations of the child’s progress is recommended. Encourage parents to work closely with the child’s teacher, guidance counselor, or school psychologist. Children with ADHD tend to perform better in school when given structure, a predictable routine, checked homework, learning aids, and resource room time.5 Specific remediation plans are recommended for comorbid learning disorders, found in approximately one-third of individuals with ADHD.
Adults with ADHD may need to modify their school or work settings to function well. College students should be encouraged to use their school’s study center, and may require accommodations for taking examinations.
Focused cognitive behavioral therapies have shown benefit in children, adolescents, and adults with ADHD.14 Training children and their parents in behavioral modification can help control the child’s disruptive behaviors, inflexibility, anxiety, or outbursts. Other useful adjuncts to treatment include remediation to improve interpersonal skills and coaching to address organization and study skills.
Pharmacotherapy
Medications are fundamental in treating ADHD1 (Table 2). In fact, a 14-month, multisite study demonstrated that medication management of ADHD was the most important variable in outcome when patients received combined pharmacologic and nonpharmacologic therapies.15 Stimulants, antihypertensives, and antidepressants are used to treat ADHD symptoms. Children, adolescents, and adults with ADHD respond similarly to pharmacotherapy.16
Psychostimulants: First-line agents
Psychostimulants are first-line agents for ADHD, based in part on extensive data showing efficacy (>250 controlled trials) and safety.17,18 Stimulants are sympathomimetic drugs that increase intrasynaptic catecholamines (mainly dopamine) by inhibiting the presynaptic reuptake mechanism (amphetamine, methylphenidate, and pemoline) and releasing presynaptic catecholamines (amphetamine).19 Methylphenidate, dextroamphetamine, amphetamine compounds, and magnesium pemoline are among the most commonly used compounds in this class.
New approaches Prescribing stimulants for ADHD has changed in two fundamental ways. Frist, in the past we covered a child’s ADHD symptoms only during school hours, but we now include time after school and weekends and holidays. Second, we also are using longer-acting stimulant preparations, which recently became available. Extended-release preparations are usually preferred for lack of in-school dosing requirements, improved compliance, reduced stigma and wear-off, and lower risk of abuse or diversion—i.e., the medication being given or sold by an individual with ADHD to someone who is using it recreationally.
Short-acting compounds such as methylphenidate, D-methylphenidate, and D-amphetamine begin working within 30 to 60 minutes. Their clinical effect usually peaks 1 and 2 hours after administration and lasts 2 to 5 hours. The amphetamine compounds (e.g., Adderall) and older sustained-release methylphenidate begin working within 60 minutes, with a clinical effect that usually peaks between 1 and 3 hours and is maintained for 5 to 8 hours).
Table 2
RECOMMENDED DOSING OF PSYCHOSTIMULANTS FOR ADHD
| Medication | Starting dosage | Maximum dosage | Usual dosing (hr) |
|---|---|---|---|
| Methylphenidate (short-acting) | |||
| Ritalin | 5 mg bid | 2 mg/kg/day | tid (4 hr) |
| Dexmethylphenidate (short-acting) | |||
| Focalin | 2.5 mg bid | 1 mg/kg/day | bid (5 hr) |
| Methylphenidate (extended-release) | |||
| Concerta | 18 mg once daily | 2 mg/kg/day | Once (12 hr) |
| Metadate CD | 20 mg once daily | Once (8-9 hr) | |
| Ritalin LA | 10 mg once daily | Once (8-9 hr) | |
| Amphetamine compounds | |||
| Adderall | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid (6 hr) |
| Adderall XR | 10 mg | Once (12 hr) | |
| Dextroamphetamine | |||
| Dexedrine | 2.5 to 5 mg once daily | 1.5 mg/kg/day | bid/tid (4 hr) |
| Dex Spansule | 5 mg | bid (6 hr) | |
| Magnesium pemoline | |||
| Cylert | 37.5 mg once in the morning | 3 mg/kg/day | Once |
Newer extended-release methylphenidate products (e.g., Ritalin LA and Metadate CD), with 8 to 9 hours’ duration of action, were developed to approximate twice-daily short-acting methylphenidate. The Concerta brand of methylphenidate, with 10 to 12 hours’ duration of action, approximates short-acting methylphenidate given three times daily. The extended-release Adderall XR brand of amphetamine compound, with a 10- to 12-hour duration of action, is similar to twice-daily Adderall.
Methylphenidate is the most studied, but among the available stimulants the literature suggests more similarities than differences in patient response.17,18 Because of the agents’ marginally different mechanisms of action, however, some patients who do not respond satisfactorily to one stimulant or manifest adverse effects may respond more favorably to another agent of this type.
Start stimulants at the lowest available dose and increase every 3 to 4 days until a response is noted or adverse effects emerge. Dose-response data indicate more robust response at higher dosages of stimulants; therefore, efficacy—rather than onset of side effects—should guide titration to an optimal dose.
Predictable short-term adverse effects include reduced appetite, insomnia, edginess, and GI upset.20 To manage these effects, consider when they occur:
- Within 2 hours after administration may signal the need to reduce the dose or change to another preparation.
- Within 4 to 6 hours after administration (e.g., moodiness) suggests the need for a longer-acting preparation or low dosing prior to the anticipated wear-off.
For insomnia, strategies include using a shorter-acting stimulant preparation, reducing the stimulant load in the afternoon, or providing adjunct treatment for the insomnia (i.e., clonidine, imipramine, mirtazapine).17 Edginess and headaches—more common in adolescents and adults—can be reduced with low-dose beta blockers. For diminished appetite in youths, caloric intake can be enhanced with a hearty breakfast, late-afternoon and evening snacks, and caloric supplements. Appetite enhancers such as cyproheptadine given nightly may be considered. Pemoline may rarely cause hepatitis and requires liver function monitoring.
Chronic use of stimulants is controversial.17,18 Although stimulants may produce anorexia and weight loss, their effect on a youth’s ultimate height is less certain. Initial reports of a persistent stimulant-associated growth decrease have not been substantiated. Other studies suggest that growth deficits may represent maturational delays related to ADHD rather than to stimulant treatment.21
Stimulants may precipitate or exacerbate tic symptoms in children with ADHD. Recent work suggests that stimulants can be used safely in youth with tic disorders,22 although up to one-third may experience worsening of tic symptoms.
Despite case reports of stimulant misuse, there is little data to support stimulant abuse among treated children with ADHD.13 However, the diversion of stimulants to youth without ADHD is a concern.
Antidepressants
Antidepressants are generally considered second-line drugs for ADHD.1,16 Bupropion, an antidepressant with indirect dopamine and noradrenergic effects, has been shown effective in ADHDin controlled trials of both children and adults.23,24
Bupropion is often prescribed first for complex patients with ADHD and substance abuse or an unstable mood disorder because of its ability to reduce cigarette smoking and improve mood, lack of monitoring requirements, and few adverse effects. Dosing is typically initiated at 100 mg of the sustained-release preparation and increased weekly to a maximum of 300 mg in younger children and 400 mg in older children or adults (i.e., 200 mg bid). Adverse effects include insomnia, activation, irritability, and (rarely) seizures.
The tricyclic antidepressants (TCAs) used in ADHD—imipramine, desipramine and nortriptyline—block the reuptake of neurotransmitters including norepinephrine. TCAs are effective in controlling abnormal behaviors and improving cognitive impairments associated with ADHD, but less so than the stimulants. TCAs are particularly useful when:
- stimulants fail to control ADHD symptoms;
- oppositional behavior, anxiety, tics, or depressive symptoms coexist within ADHD or occur during its treatment.
Desipramine appears to be the most effective TCA for ADHD, followed by nortriptyline and imipramine.25,26 TCAs are dosed starting with 25 mg/d and slowly increased to a maximum of 5 mg/kg/day (2 mg/kg/day for nortriptyline). Although immediate relief can be seen, a delay of up to 6 weeks for maximal effect is common. Typical adverse effects include dry mouth, constipation, sedation, and weight gain.
Four deaths have been reported in children with ADHD treated with desipramine; however, independent evaluation of these cases failed to support a causal link. As minor increases in heart rate and ECG intervals are predictable with TCAs, ECG monitoring at baseline and at therapeutic dosages is recommended.
Although serotonin reuptake inhibitors are not generally useful for ADHD, venlafaxine appears to have mild efficacy, perhaps because of its dose-dependent noradrenergic reuptake inhibition.27
Monoamine oxidase inhibitors (MAOIs) have been shown effective in juvenile ADHD. Response to treatment is rapid, and standard antidepressant dosing is often necessary.16 A major limitation to the use of MAOIs is the potential for hypertensive crisis associated with dietetic transgressions and drug interactions.
Other treatment options
Antihypertensives The antihypertensive agents clonidine28 and guanfacine29 are used to treat the hyperactive-impulsive symptoms of ADHD in youth. Clonidine is relatively shortacting, with usual daily dosage ranges from 0.05 to 0.4 mg.28 Guanfacine is longer acting and less potent, with usual daily dosage ranges from 0.5 to 4 mg.29
Antihypertensives have been used to treat ADHD and associated tics, aggression, and sleep disturbances, particularly in younger children.16 Although sedation is more common with clonidine than guanfacine, both agents may cause depression and rebound hypertension. Cardiovascular monitoring (vital signs, ECG) remains optional.
New agents Novel compounds, along with new preparations and delivery systems of existing stimulant medications, are being investigated for managing ADHD. New agents are being tested in adults with ADHD because adults and youth respond similarly to ADHD medications, and there are ethical concerns about drug testing in children.
Atomoxetine, a noradrenergic reuptake inhibitor under development, has been shown in open and controlled studies of adults and youth30 to be effective in treating ADHD. Atomoxetine appears well tolerated, with no blood monitoring requirements.
Cholinergics and genes Selective use of cholinergic agents (e.g., donepezil) may also be helpful for the cognitive dysfunction in ADHD,24 either as monotherapy or in combination with other agents for ADHD. Multiple centers are investigating the possible link between response to pharmacologic therapy and ADHD genotype.
Combination therapy
Combinations of pharmacologic agents can be used to treat comorbid ADHD, to augment response to a single agent, for pharmacokinetic synergism, and to manage adverse effects that emerge during treatment. Examples include:
- a tricyclic antidepressant and a stimulant to heighten response to treatment;
- an antidepressant plus a stimulant for ADHD and comorbid depression;
- adjunctive use of clonidine for sleep or to manage aggressive behavior;
- use of mood stabilizers with ADHD medications for comorbid bipolar disorder.16
Pharmacologic intervention for prominent concomitant mood disorders (depression and bipolarity) and anxiety should be sequenced prior to ADHD treatment.
Summary of treatment recommendations
Based on efficacy and safety, stimulants are first-line agents for routine management of ADHD, followed by antidepressants and antihypertensives. Patients who do not respond to the initial stimulant or who manifest adverse effects should be considered for a trial with an alternate stimulant. If two stimulant trials are unsuccessful, bupropion and the tricyclic antidepressants are reasonable second-line agents.
Antihypertensives alone or in combination with other ADHD medication may help youths with tics,31 prominent hyperactivity, impulsivity, or aggressiveness. MAOIs may be considered for refractory patients, and cholinergic agents (e.g., donepezil) may be used for excessive cognitive difficulties such as organization, planning, and time management.
Related resources
- Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: The Guilford Press, 1998.
- Wilens T. Straight Talk About Psychiatric Medications for Kids. New York: The Guilford Press, 1998.
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD), www.chadd.org
Drug brand names
- Atomoxetine • (under development)
- Bupropion • Wellbutrin
- Clonidine • Catapress
- Dextro-amphetamine • Dexedrine
- Dexmethylphenidate • Focalin
- Donepezil • Aricept
- Guanfacine • Tenex
- Methylphenidate • Ritalin, Concerta, Metadate
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
Dr. Biederman reports that he receives research/grant support from, and is on the speaker’s bureau and advisory boards of Eli Lilly & Co. and Shire Laboratories. He also reports that he receives research/grant support from Wyeth-Ayerst Pharmaceuticals, Pfizer Inc., Cephalon Pharmaceutical, Janssen Pharmaceutica, and Noven Pharmaceutical; is on the speaker's bureau of GlaxoSmithKline, Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Alza/McNeil Pharmaceutical and Cephalon Pharmaceutical; and is on the advisory board of Cell Tech, Noven Pharmaceutical, and Alza/McNeil Pharmaceuticals.
Drs. Wilens and Spencer report that they receive research/grant support from, are on the speakers bureau of, and/or serve as consultants to Abbott Laboratories, McNeil Pharmaceuticals, Celltech Medieva, GlaxoSmithKline, Eli Lilly & Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth-Ayerst Pharmaceuticals.
1. Goldman L, Genel M, Bezman R, Slanetz P. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA 1998;279:1100-7.
2. Hechtman L, Weiss G. Controlled prospective fifteen-year follow-up of hyperactives as adults: mon-medical drug and alcohol use and anti-social behaviour. Can J Psychiatry 1986;31:557-67.
3. Fischer M. Persistence of ADHD into adulthood: it depends on whom you ask. The ADHD Report 1997;5:8-10.
4. Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 1998;59:4-16.
5. Barkley R. Attention-deficit/hyperactivity disorder: A handbook for diagnosis and treament (2nd ed). New York: Guilford Press, 1998.
6. Zametkin A, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 1998;59:17-23.
7. Dougherty D, Bonab A, Spencer T, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999;354:2132-3.
8. Faraone SV, Biederman J, Weiffenbach B, et al. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:768-70.
9. Millstein RB, Wilens TE, Biederman J, Spencer TJ. Presenting ADHD symptoms and subtypes in clincially referred adults with ADHD. J Attent Disord 1997;2:159-66.
10. Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 1991;148:564-77.
11. Woznia J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis 1997;185:475-82.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone S. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
14. Abikoff H. Cognitive training in ADHD children; less to It than meets the eye. J Learn Disabil 1991;24:205-9.
15. Group MTS. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-86.
16. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention deficit disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 1996;35:409-32.
17. Wilens T, Spencer T. The stimulants revisited. In: Stubbe C. Child an adolescent psychiatric clinics of North America. Philadelphia: JB Saunders, 2000;573-603
18. Greenhill L, Osman B. Ritalin: theory and practice. New York: Mary Ann Liebert, 1999.
19. Elia J, Borcherding BG, Potter WZ, et al. Stimulant drug treatment of hyperactivity: biochemical correlates. Clin Pharmacol Ther 1990;48:57-66.
20. Barkley RA, McMurray MB, Edelbrock CS, Robbin K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86:184-92.
21. Spencer TJ, Biederman J, Harding M, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry 1996;35:1460-9.
22. Gadow K, Sverd J, Sprafkin J, Nolan E, Grossman S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56:330-6.
23. Conners CK, Casat CD, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 1996;35:1314-21.
24. Wilens T, Biederman J, Spencer T, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:1931-7.
25. Biederman J, Baldessarini RJ, Wright V, Knee D, Harmatz JS. A double-blind placebo controlled study of desipramine in the treatment of ADD. I. Efficacy. J Am Acad Child Adolesc Psychiatry 1989;28:777-784.
26. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2000;10:193-204.
27. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/ hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57:184-9.
28. Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Adolesc Psychiatry 1985;24:617-29.
29. Horrigan JP, Barnhill LJ. Guanfacine for treatment of attention-deficit hyperactivity disorder in boys. J Child Adolesc Psychopharmacol 1995;5:215-23.
30. Kratochvil CJ, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2001;11:167-70.
31. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics. A ramdomized controlled trial. Neurology 2002;58(4):527-36.
1. Goldman L, Genel M, Bezman R, Slanetz P. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA 1998;279:1100-7.
2. Hechtman L, Weiss G. Controlled prospective fifteen-year follow-up of hyperactives as adults: mon-medical drug and alcohol use and anti-social behaviour. Can J Psychiatry 1986;31:557-67.
3. Fischer M. Persistence of ADHD into adulthood: it depends on whom you ask. The ADHD Report 1997;5:8-10.
4. Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 1998;59:4-16.
5. Barkley R. Attention-deficit/hyperactivity disorder: A handbook for diagnosis and treament (2nd ed). New York: Guilford Press, 1998.
6. Zametkin A, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 1998;59:17-23.
7. Dougherty D, Bonab A, Spencer T, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999;354:2132-3.
8. Faraone SV, Biederman J, Weiffenbach B, et al. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:768-70.
9. Millstein RB, Wilens TE, Biederman J, Spencer TJ. Presenting ADHD symptoms and subtypes in clincially referred adults with ADHD. J Attent Disord 1997;2:159-66.
10. Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 1991;148:564-77.
11. Woznia J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis 1997;185:475-82.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone S. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
14. Abikoff H. Cognitive training in ADHD children; less to It than meets the eye. J Learn Disabil 1991;24:205-9.
15. Group MTS. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-86.
16. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention deficit disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 1996;35:409-32.
17. Wilens T, Spencer T. The stimulants revisited. In: Stubbe C. Child an adolescent psychiatric clinics of North America. Philadelphia: JB Saunders, 2000;573-603
18. Greenhill L, Osman B. Ritalin: theory and practice. New York: Mary Ann Liebert, 1999.
19. Elia J, Borcherding BG, Potter WZ, et al. Stimulant drug treatment of hyperactivity: biochemical correlates. Clin Pharmacol Ther 1990;48:57-66.
20. Barkley RA, McMurray MB, Edelbrock CS, Robbin K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86:184-92.
21. Spencer TJ, Biederman J, Harding M, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry 1996;35:1460-9.
22. Gadow K, Sverd J, Sprafkin J, Nolan E, Grossman S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56:330-6.
23. Conners CK, Casat CD, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 1996;35:1314-21.
24. Wilens T, Biederman J, Spencer T, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry 1999;156:1931-7.
25. Biederman J, Baldessarini RJ, Wright V, Knee D, Harmatz JS. A double-blind placebo controlled study of desipramine in the treatment of ADD. I. Efficacy. J Am Acad Child Adolesc Psychiatry 1989;28:777-784.
26. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2000;10:193-204.
27. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/ hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57:184-9.
28. Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Adolesc Psychiatry 1985;24:617-29.
29. Horrigan JP, Barnhill LJ. Guanfacine for treatment of attention-deficit hyperactivity disorder in boys. J Child Adolesc Psychopharmacol 1995;5:215-23.
30. Kratochvil CJ, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2001;11:167-70.
31. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics. A ramdomized controlled trial. Neurology 2002;58(4):527-36.