User login
The exercise treadmill test: Estimating cardiovascular prognosis
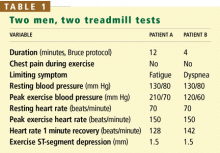
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
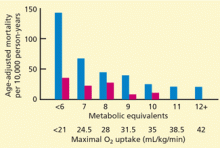
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
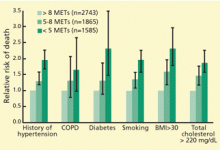
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
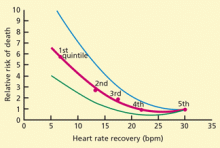
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
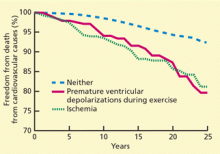
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
KEY POINTS
- Of the prognostic factors, exercise duration is the one most strongly associated with risk of coronary events and death, independent of age, sex, or known presence and severity of coronary artery disease.
- A decrease in blood pressure with exercise can reflect severe coronary artery disease or left ventricular systolic dysfunction.
- A heart rate that does not increase adequately during exercise or does not recover rapidly after exercise is associated with an increased risk of death.
- Exercise training may help to improve the prognosis of patients with an abnormal hemodynamic response to exercise caused by poor general health.