User login
Adverse events from systemic treatment of cancer and patient-reported quality of life
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
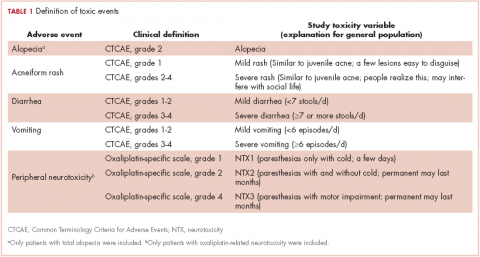
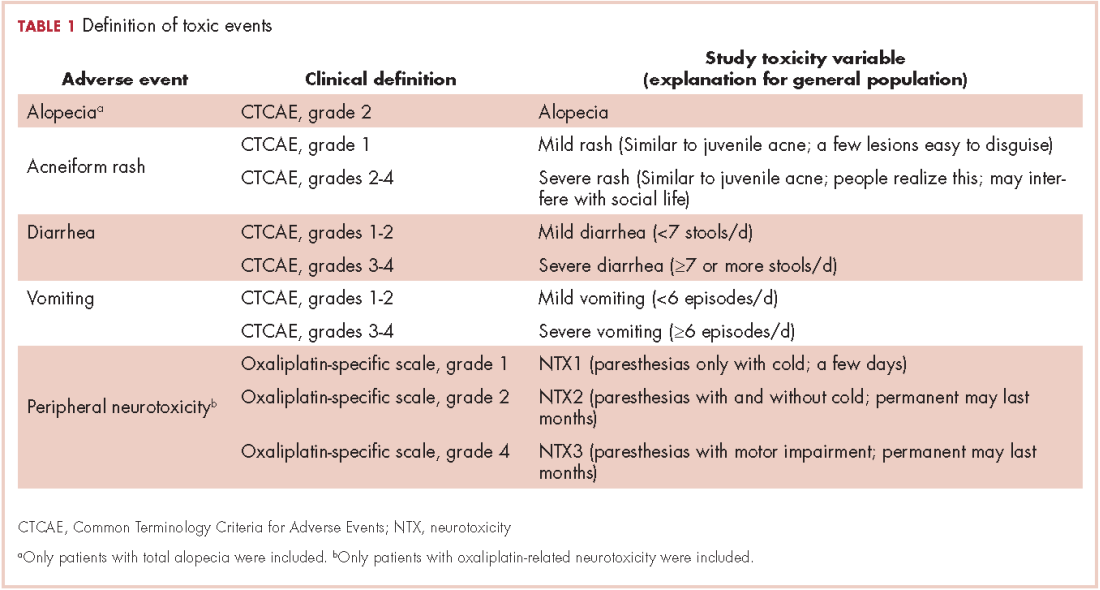
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
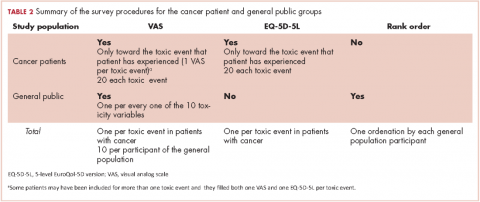
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
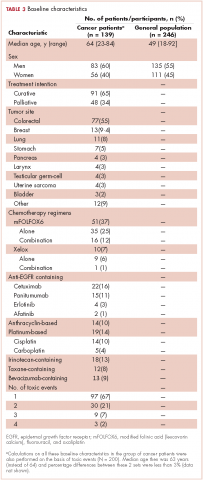
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
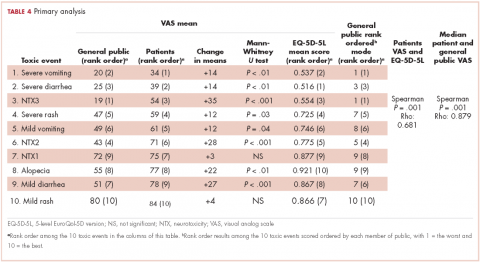
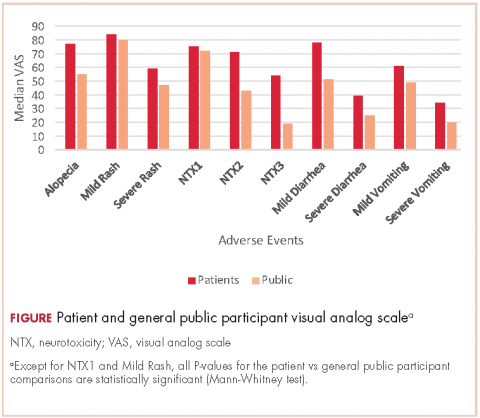
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156