User login
Measuring Restrictive Lung Disease Severity Using FEV1 vs TLC
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
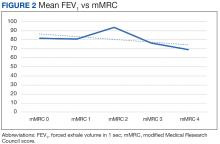
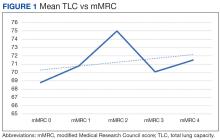
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745


 Author disclosures
Author disclosures