User login
Making sense of CYP2D6 and CYP1A2 genotype vs phenotype
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
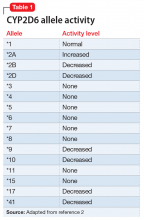
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.
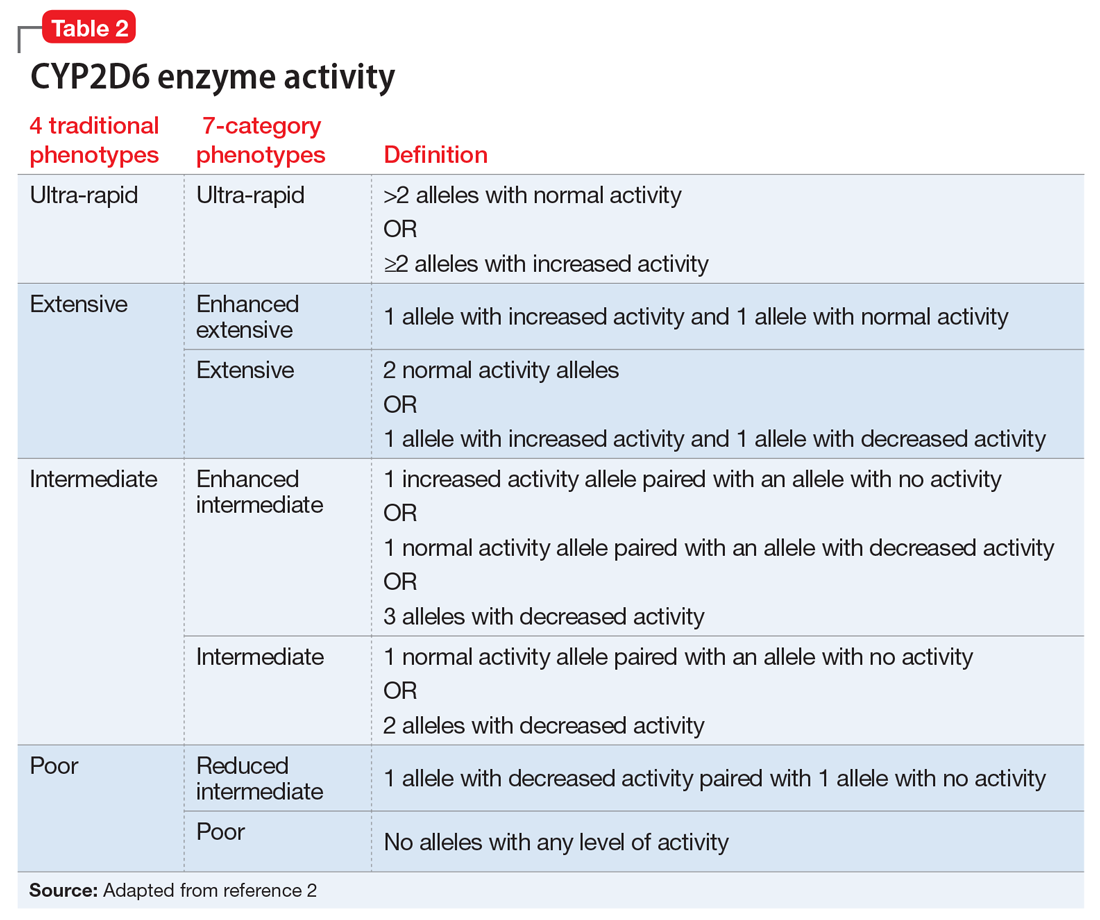
Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.
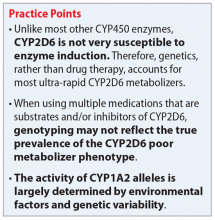
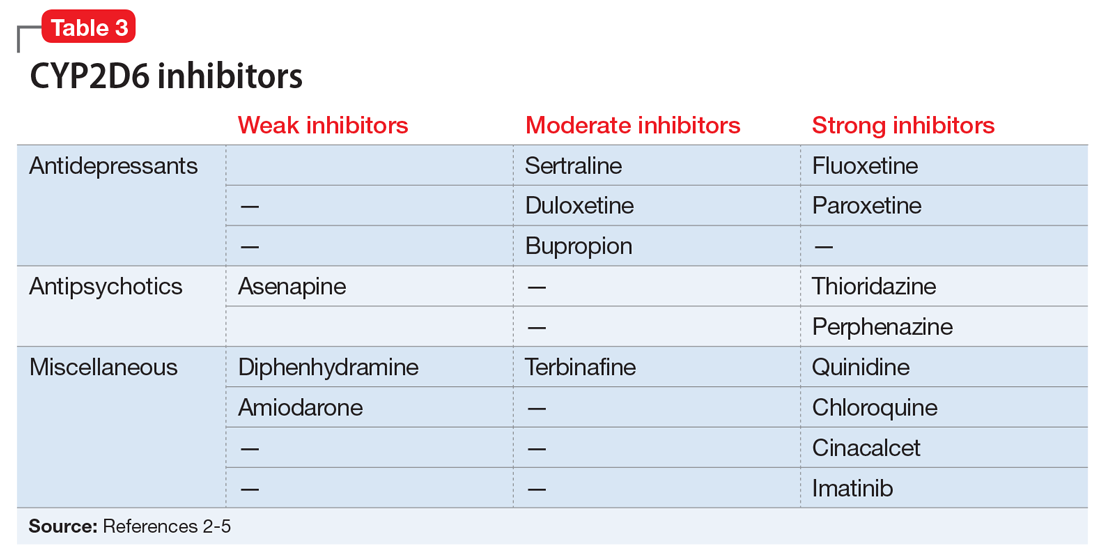
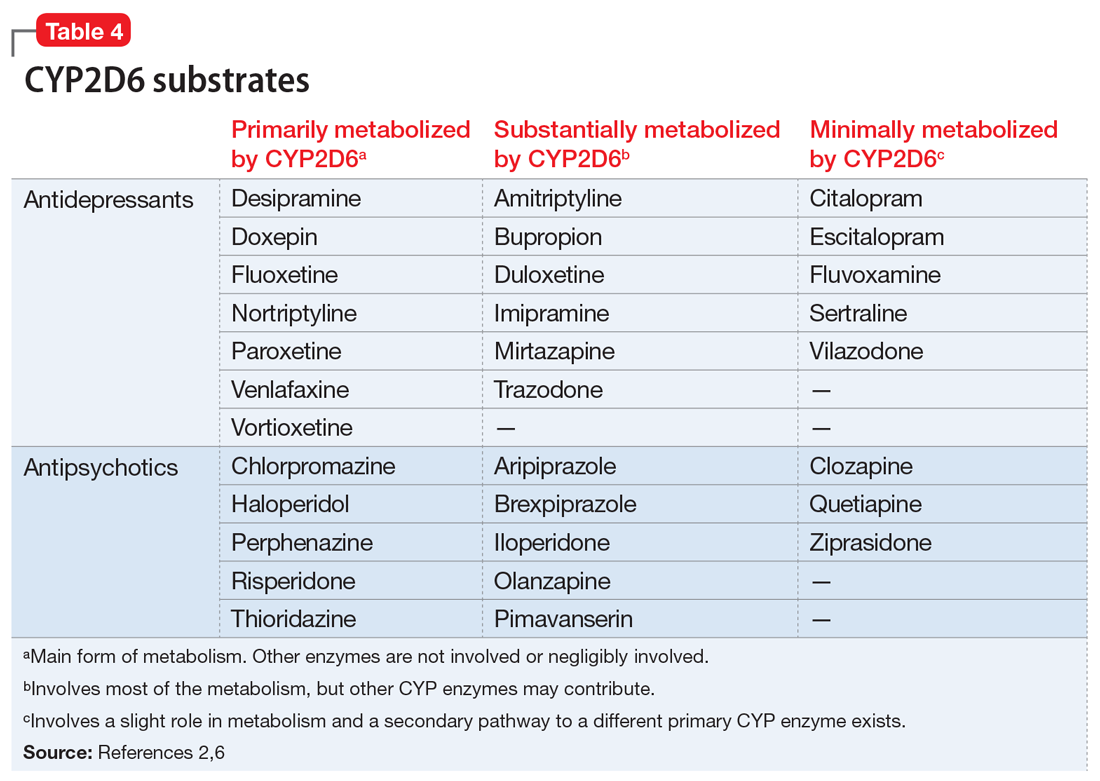
Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.
Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
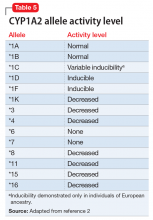
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.
Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.
Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.
Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.
Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.
Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.
Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
Aggressive and delusional about his alien origins, but refusing treatment
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
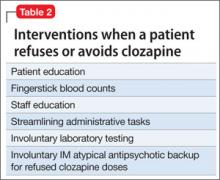
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.