User login
Highlights from the 2020 Scientific Meeting of the Society of Gynecologic Surgeons

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida
Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
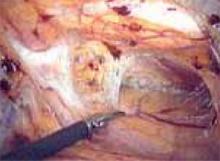
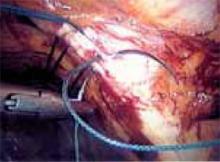
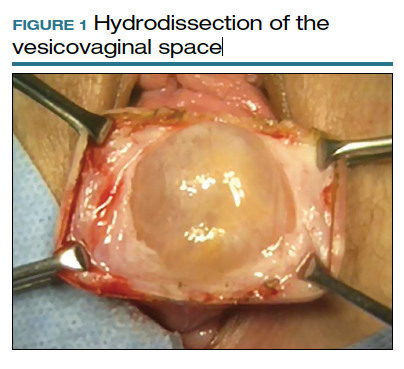
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.
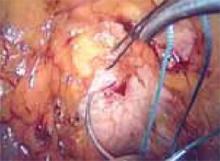
Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
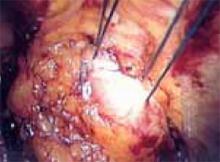
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
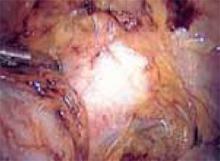
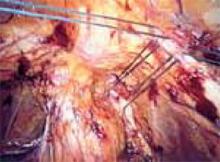
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.
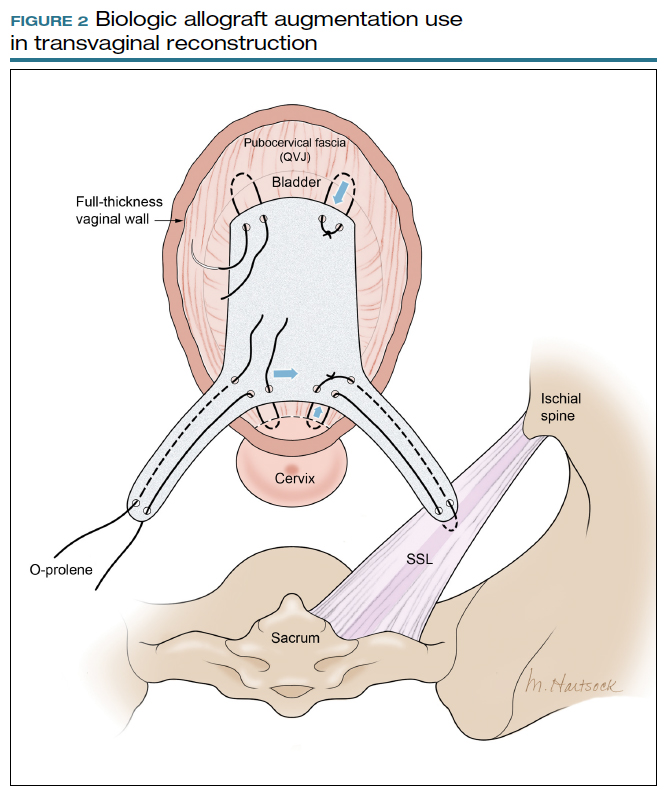
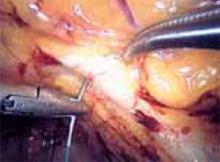
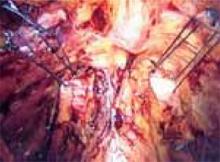
Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
Highlights from the 2018 Society of Gynecologic Surgeons Scientific Meeting
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
Laparoscopic Burch colposuspension for stress urinary incontinence: When, how, and why?
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
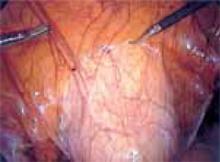
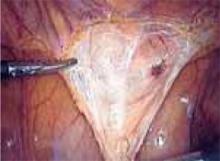
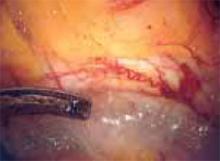
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.