User login
Off-label medications for addictive disorders
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
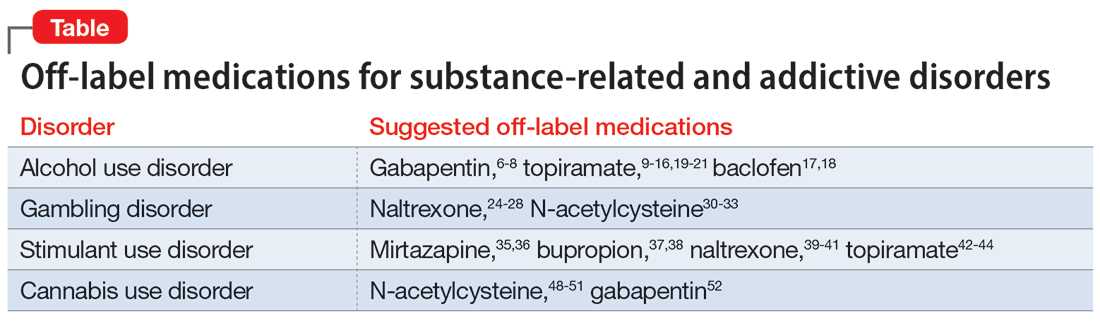
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52
The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Extended-release injectable naltrexone for opioid use disorder
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
Managing patients with comorbid opioid and alcohol use disorders
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.
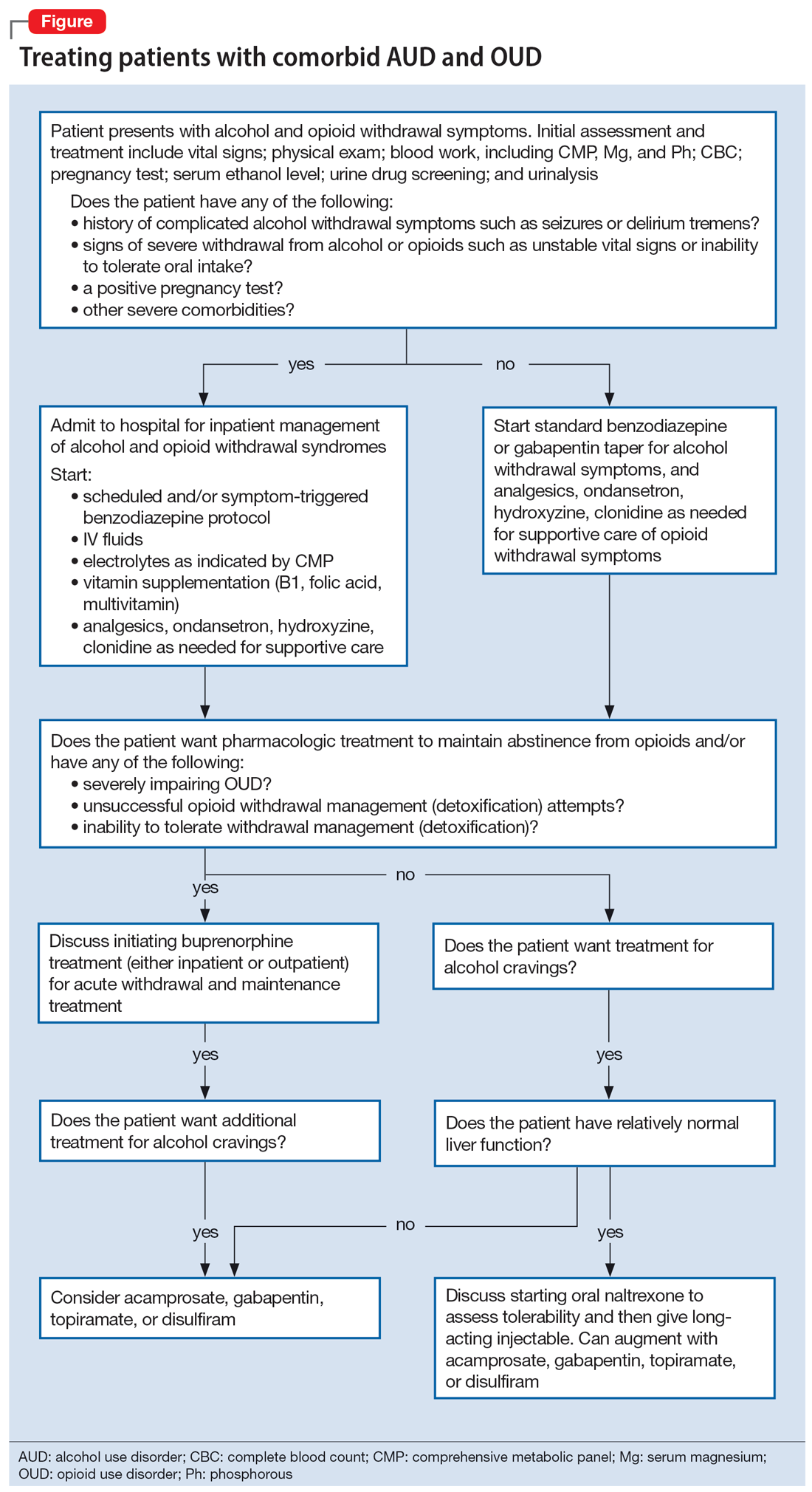
CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
Laboratory monitoring for patients on buprenorphine: 10 questions
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
Measuring cotinine to monitor tobacco use and smoking cessation
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.