User login
What clinicians need to know about treating opioid use disorder
Communities across the United States have experienced a near-epidemic of opioid abuse. Deaths from opioid overdose have doubled since 2000 and increased 14% from 2013 to 2014.1 Treatment strategies for opioid use disorder (OUD) target individual well-being with the goal of preventing relapse. Most treatment approaches help patients gain self-confidence and have been described by patients as “giving them their life back.”
Broadly, the major phases of treatment for OUD are similar to those for other substance use disorders, and involve acute detoxification followed by long-term maintenance of sobriety. There are 3 main phases of treatment for OUD:
- treatment engagement
- stabilization and harm reduction
- sustained abstinence.
Long-term maintenance of sobriety in OUD could involve medications that are FDA-approved for this indication: methadone, buprenorphine, and naltrexone. Psychosocial interventions can be used individually and in combination with pharmacotherapy. Because OUD is a chronic disorder typically characterized by intermittent relapse, patients could move back and forth between the different phases of treatment.
In this article, we highlight the medication and non-medication treatment options for the long-term management of OUD.
Defining abuseExogenous opioids are synthetic substances used for their analgesic and morphine-like properties. Opioids are indicated for the treatment of certain pain conditions and exist in varying potencies and delivery systems, which are tailored for specific types of pain.2 Opioids work through activity at opioid receptors found in the brain, spinal cord, gut, and other organs. Agonism of specific opioid receptors results in a decreased perception of pain and contributes to their abuse potential.
Because of their abuse potential, prescription of opioids is governed by the Controlled Substances Act3 of the United States. Despite these regulations, approximately 4.5% of U.S. adults from a nationally representative sample were found to be misusing prescription opioids.4 Another study used data from the National Survey on Drug Use and Health and showed an increasing prevalence of OUD involving prescription drugs and resulting in increased mortality.5 The mortality rate from prescription opioids was found to be higher than for all other illicit drugs combined in 2013.6 Recently, Congress passed the Comprehensive Addiction and Recovery Act, which is intended to address the opioid crisis by expanding access to treatments for opioid overdose and addiction treatment services.7
The term “opioid use disorder” is found in DSM-58 and replaces the previous diagnoses of opioid abuse and dependence in DSM-IV-TR.9 OUD is characterized by a strong desire to continue using opioids despite problems associated with their use. Patients with OUD often experience cravings for opioids, tolerance, repeated failures at cutting down or limiting use, and decreased involvement in social activities.
Maladaptive use of opioids can result in physiologic dependence and lead to withdrawal symptoms, including anxiety, drug craving, insomnia, rhinorrhea, lacrimation, diarrhea, and piloerection. However, physiologic dependence might not necessarily lead to development of OUD, which can be diagnosed when enough other diagnostic criteria present in the absence of physiologic dependence.
Misuse of prescription opioids is more prevalent among patients who meet criteria for other substance use disorders than among patients who do not.10 Misuse of prescription opioids often results in greater health care utilization, including the need for emergency services, hospitalization, and detoxification.
Assessing for OUDThe Addiction Severity Index was designed to classify and monitor misuse of opioids. The Index has poor sensitivity; it detects recent opioid use but fails to differentiate patients using prescribed opioids from those who are abusing opioids.11 By comparison, the Current Opioid Misuse Measure (COMM) was developed to monitor opioid use in chemical dependency treatment settings.12 The COMM has reliable internal consistency13 and validity and can be used to assess use of opioids outside of routine medical care. To gauge the severity of withdrawal symptoms, the Objective Opioid Withdrawal Scale or Clinical Opiate Withdrawal Scale14 can be used. Table 1 summarizes some standardized tools used to assess OUD.
Neurobiological considerationsOpioids work through activity at mu, kappa, and delta opioid receptors. These receptors are present in both the peripheral and the central nervous system. Exogenous opioids work through activity at G protein-coupled receptors and by activating specific neurotransmitter systems. The effect of a given opioid drug is dependent on the type and location of receptors it modulates and can range from CNS depression to euphoria.
For example, mu receptor activation produces sedation, euphoria, or analgesia depending upon the location, frequency, and duration of receptor occupancy. Activation of CNS mu receptors can cause miosis and respiratory depression, whereas mu receptor activation in the peripheral nervous system can cause constipation and cough suppression. Mu receptor stimulation through various G-proteins triggers the second messenger cascade, generating enzymes such as cyclic adenosine monophosphate.
Clinical considerationsTreating OUD is challenging because of the ease with which patients can obtain opioids and because sometimes OUD occurs iatrogenically. Engaging patients in treatment is an important step in recovery, but it does not necessarily lead to reduction in opioid use. The engagement stage can involve outreach workers to encourage further treatment. Developing a therapeutic alliance and appropriately incentivizing patients also promotes entry into treatment. Motivational interviewing is used often in substance use treatment programs and can help engage patients in treatment and evaluate their willingness to change problematic behaviors.
Managing acute withdrawal symptoms. Withdrawal symptoms usually are not life threatening, but can be in the context of other medical conditions, such as autonomic instability, hypertension, cardiovascular disease, and dehydration. Withdrawal symptoms also can be life-threatening during in utero exposure to a fetus. Pharmacotherapeutic options to treat opioid withdrawal symptoms include long-acting opioids, such as methadone and buprenorphine,15 which can be administered in an ambulatory setting. The combination of buprenorphine and naloxone also can be used to treat opioid withdrawal symptoms.
The alpha-2 agonist16 clonidine, although not FDA-approved for OUD or opioid withdrawal, could be used to shorten the duration of withdrawal symptoms. Clonidine also decreases methadone withdrawal and can be combined with naloxone to target naloxone-induced opioid withdrawal symptoms.17,18 Nalbuphine and butorphanol should be avoided during opioid withdrawal because they antagonize opioid receptors and can precipitate withdrawal symptoms.19,20
Maintenance phase involves long-term stabilization and relapse prevention. Treatment options include medication and non-medication interventions.21
Non-pharmacologic treatment options,22 principally psychosocial interventions, can be used on their own or in combination with medications for maintenance treatment of OUD. Psychosocial interventions include structured, professionally administered interventions such as cognitive-behavioral therapy (CBT), aversion therapy, and day-treatment programs. Interventions such as peer counseling and self-help groups also are considered psychosocial interventions, but do not require the same type of professional training.
Peer support groups such as Narcotics Anonymous (NA) help members achieve and maintain sobriety and often focus on a traditional 12-step format or on the more recent Matrix Model,23,24 which is an intensive outpatient treatment program based on components of relapse prevention, motivational interviewing, CBT, and psychoeducation.
In these peer support models, group members discuss patterns of substance use and help one another recognize and overcome problematic behaviors. Groups may vary in terms of their specific approach. For example, NA encourages group members to focus on addiction itself while Methadone Anonymous prefers participants also discuss pharmacologic treatment experiences. Additional services for finding housing and assisting with job placement are also part of some relapse prevention strategies.
Although studies on the use of abstinence-based treatments are limited, abstinence-based therapy is an option for patients wishing to undergo chemical dependency treatment without taking prescription medications to address cravings or withdrawal symptoms.25 However, abstinence-based treatments have been shown to be less effective in improving outcomes than medication-assisted treatment (MAT).26 MAT combines medications and behavioral therapies for treating substance use disorders.
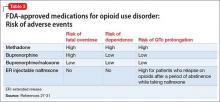
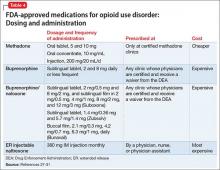
Pharmacologically, OUD can be treated with opioid agonist and antagonist medications. As summarized in Tables 2-4,27-31 these medications differ based on their pharmacokinetic and pharmacodynamic profiles and intrinsic activity at mu opioid receptors. Opioid system agonists, such as methadone and buprenorphine, decrease cravings by mimicking the activity of exogenous opioids. The opioid antagonists naloxone and naltrexone reinforce abstinence by inhibiting the euphoric effects associated with opioid use. The medication of choice for a given patient depends on:
- treatment adherence
- clinical setting
- degree of withdrawal symptoms
- motivation.26
If a patient is actively seeking abstinence from opioids, either agonist or antagonist treatment can be used. In cases where a patient is not seeking abstinence, then preference should be given to opioid agonists to prevent overdose.26
Evidence suggests MAT can improve outcomes with OUD when compared with abstinence treatment alone. Several randomized, controlled, trials showed methadone and buprenorphine were more effective at treating OUD compared with treatment without medication. To date, 3 medications have been FDA-approved for treating OUD: methadone, buprenorphine, and naltrexone.26 All 3 medications differ in their pharmacokinetic and pharmacodynamic profiles and intrinsic activities at central mu-opioid receptor, as summarized in Tables 2-4.27-31
MethadoneMethadone reduces the euphoric effects of opioid use because it binds to and blocks opioid receptors. Methadone is an opioid replacement strategy; higher dosages are used for maintenance treatment to prevent additional dosages of opioids from causing euphoria. Methadone typically is administered once daily. However, in certain circumstances, such as rapid metabolism or pregnancy, it can be given as a twice-daily dosing regimen. Specific ABCB1 variants and DRD2 genetic polymorphisms (simultaneous occurrence of ≥2 genetically determined phenotypes) might determine the dosage requirements of methadone.32
Methadone during pregnancy. Methadone is the treatment of choice for opioid-dependent women during pregnancy33 and is listed as pregnancy category C because it can result in physiologic dependence of the newborn, although there are no documented controlled studies in humans to assess this risk. Methadone can be used while breast-feeding as long as patients are HIV-negative and not abusing other drugs.34,35 Because the methadone concentration in breast milk generally is low, the medication can be administered to nursing mothers after a careful consideration of risks and benefits.
Methadone administration. There are stringent eligibility criteria for methadone administration; not all physicians are authorized to prescribe methadone. Its use is federally regulated and only licensed treatment programs and licensed inpatient detoxification units can prescribe and dispense methadone in controlled settings and under the direct supervision of clinical personnel (Table 5).36 Patients meeting eligibility criteria can attend a specialized methadone clinic.
One of the challenges when using methadone for long-term management of OUD is tapering the dosage and attempting to discontinue the medication. Discontinuation of methadone leads to withdrawal symptoms and requires a carefully tailored tapering schedule. The literature on methadone tapering is limited. Tapering schedules could differ from practice to practice and, in many cases, are highly individualized based on the need and response of specific patients.
Dosage reduction schedules can last from 2 to 3 weeks to 6 months. Studies indicate rapid reduction worsens treatment outcomes and protracted tapering is associated with better outcomes. A suggested tapering schedule could involve decreasing the dosage by 20% to 25% until reaching a dosage of 30 mg/d, then decreasing by 5 mg/d every 3 to 5 days until reaching a dosage of 10 mg/d, before finally decreasing by 2.5 mg/d every 3 to 5 days.
Some randomized trials have shown better outcomes with long-term treatment. The goal of many programs is transitioning from maintenance treatment to abstinence. However, programs targeting maintenance rather than abstinence have been shown to be more effective.
The FDA has no defined limits for treatment duration with either methadone or buprenorphine. Therefore, the decision to taper or discontinue either medication should be made carefully case by case, using sound clinical judgment. Studies show that methadone treatment could reduce the spread of HIV,37,38 decrease criminal behaviors,39 and reduce overall mortality rates.40 A follow-up study comparing individuals randomly assigned to receive methadone or buprenorphine for OUD showed reduced risk of mortality overall40 in both groups.
Adverse events reported during treatment with methadone include decreased libido, erectile dysfunction, constipation, drowsiness, QTc prolongation, and torsade de pointes.41 Therefore, the FDA recommends obtaining a detailed medical history and baseline electrocardiogram (ECG), with a repeat ECG within the first month of treatment and then annually. Informing patients about the possibility of arrhythmias is part of the informed consent process before starting methadone.
Clinicians also should be vigilant when using methadone in combination with other medications that can prolong the QTc interval (eg, some antipsychotics). Methadone has a greater risk of fatal overdose then buprenorphine. A large-scale study of >16,000 patients reported a 4-fold increase in mortality resulting from methadone overdose compared with buprenorphine.42
BuprenorphineBuprenorphine is a partial opioid agonist at the mu opioid receptor. A full opioid agonist binds and fully activates the opioid receptors; an antagonist blocks the same. An opioid receptor partial agonist partially activates the receptor. Therefore, an opioid system partial agonist is a functional antagonist and, at lower dosages, has weak agonist effects; at higher dosages, a partial agonist antagonizes other endogenous and exogenous opioids that compete for binding at the same receptor.43 Because of the partial agonist effect, buprenorphine could result in less physical dependence and less withdrawal symptoms.
Administration. In contrast to methadone, buprenorphine can be prescribed by physicians for long-term management of OUD in the United States. Buprenorphine is available in 2 formulations: a sublingual form for daily use and a long-acting form that causes less withdrawal symptoms and cravings. In May 2016 the FDA approved the first buprenorphine implant for use in opioid dependence.44,45
To prevent withdrawal symptoms, a 24-hour period of opioid abstinence is recommended before starting buprenorphine or buprenorphine/naloxone treatment.46 Although lacking empirical evidence, catechol-O-methyltransferase (COMT) inhibitors, such as entacapone, have an anti-craving affect and are used by some clinicians to improve adherence with buprenorphine. This is because of their ability to balance dopamine, which is central to the reward pathway responsible for cravings. Although use of COMT inhibitors might make sense intuitively, such use is off-label and should be based on clinical judgment and a review of the available literature. A study showed that tapering buprenorphine for 4 weeks in combination with naltrexone improved the abstinence rate.47
Adverse effects. Some of the adverse events reported during treatment with buprenorphine include fever, back pain, nausea, cough, sedation, difficulty with urination, and constipation. Respiratory depression is a less common effect of buprenorphine, compared with full opioid agonists, because of the medication’s mechanism of action as a partial agonist.48 As a result, buprenorphine has been shown to have a lower risk of fatal overdose than methadone.49 Studies have shown buprenorphine to be more likely than methadone to reduce neonatal abstinence syndromes.50
NaltrexoneNaltrexone is an opioid antagonist and is an option to promote relapse prevention. Because of its antagonist properties, naltrexone treatment should always start after opioid detoxification because it can potentiate immediate withdrawal symptoms. Naltrexone is available in oral and long-acting formulations, the latter of which may be considered in patients who have difficulty with adherence.37
Oral naltrexone is taken as a single 50 mg-tablet once daily, whereas dosing for long-acting naltrexone in injectable and implantable formulations varies. These long-acting naltrexone formulations typically are administered monthly. Some of the adverse events reported during treatment with naltrexone are nausea, liver damage, and injection site pain.51
Buprenorphine/naloxoneBecause of buprenorphine’s agonist effects, it has a relatively high abuse potential compared with other opioids.52 Naloxone, on the other hand, is an opioid antagonist and is poorly absorbed when given orally and is associated with withdrawal symptoms if used intravenously. Therefore, naloxone is added to buprenorphine to decrease the likelihood of abuse when both are used as a combination product.53
Buprenorphine is combined with naloxone in a ratio of 4:1. Induction begins by using a 2 mg/0.5 mg tablet with dosage titration until symptoms abate. A combination of buprenorphine and naloxone also is available in film and tablet formulations. Patients must abstain from other opioids for at least 24 hours before initiating buprenorphine/naloxone treatment to prevent the precipitation of withdrawal symptoms.
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
2. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695-1700.
3. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
4. Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94(1-3):38-47.
5. Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015;314(14):1468-1478.
6. Centers for Disease Control and Prevention. National Center for Health Statistics, 2014. Multiple cause of death data. http://wonder.cdc.gov/mcd.html.
7. Twachtman G. Congress sends opioid legislation to the President. Clinical Psychiatry News. http://www.clinicalpsychiatrynews.com/?id=2407&tx_ttnews[tt_news]=524025&cHash=e93d5d1f86d20e53d3e2d8b07e9562b2. Published July 15, 2016. Accessed July 18, 2016.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
10. McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav. 2008;33(10):1297-1305.
11. Butler SF, Villapiano A, Malinow A. The effect of computer-mediated administration on self-disclosure of problems on the Addiction Severity Index. J Addict Med. 2009;3(4):194-203.
12. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152(2):397-402.
13. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26(9):770-776.
14. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259.
15. Fiellin DA, O’Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347(11):817-823.
16. Gowing LR, Farrell M, Ali RL, et al. α2‐Adrenergic agonists in opioid withdrawal. Addiction. 2002;97(1):49-58.
17. Loimer N, Hofmann P, Chaudhry H. Ultrashort noninvasive opiate detoxification. Am J Psychiatry. 1993;150(5):839.
18. Charney DS, Sternberg DE, Kleber HD, et al. The clinical use of clonidine in abrupt withdrawal from methadone. Effects on blood pressure and specific signs and symptoms. Arch Gen Psychiatry. 1981;38(11):1273-1277.
19. Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1989;248(3):929-937.
20. Preston KL, Bigelow GE, Liebson IA. Discrimination of butorphanol and nalbuphine in opioid-dependent humans. Pharmacol Biochem Behav. 1990;37(3):511-522.
21. Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280(22):1936-1943.
22. Amato L, Minozzi S, Davoli, M, et al. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4.
23. Obert JL, McCann MJ, Marinelli-Casey P, et al. The matrix model of outpatient stimulant abuse treatment: history and description. J Psychoactive Drugs. 2000;32(2):157-164.
24. Mayet S, Farrell M, Ferri M, et al. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2005:CD004330.
25. McAuliffe WE. A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. J Psychoactive Drugs. 1990;22(2):197-209.
26. Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
27. Gibson AE, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug Alcohol Rev. 2007;26:405-410.
28. Clark L, Haram E, Johnson K, et al. Getting started with medication-assisted treatment with lessons from advancing recovery. Madison, WI: University of Wisconsin-Madison; 2010.
29. U.S. Food and Drug Administration. Vivitrol (naltrexone for extended-release injectable suspension): NDA 21-897C—Briefing document/background package. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharmacologic DrugsAdvisoryCommittee/UCM225664.pdf. Published September 16, 2010. Accessed July 11, 2016.
30. Providers Clinical Support System. PCSS Guidance. Buprenorphine induction. http://pcssmat.org/wp-content/uploads/2014/02/PCSS-MATGuidanceBuprenorphineInduction.Casadonte.pdf. Updated November 27, 2013. Accessed July 18, 2016.
31. An introduction to extended-release injectable naltrexone for the treatment of people with opioid dependence. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. HHS Publication No. (SMA) 12-4682.
32. Doehring A, von Hentig N, Graff J, et al. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19(6):407-414.
33. Mitchell JL. Pregnant, substance-abusing women: treatment improvement protocol (TIP) Series 2. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1993. DHHS Publication No. (SMA) 95-3056.
34. McCarthy JJ, Posey BL. Methadone levels in human milk. J Hum Lact. 2000;16(2):115-120.
35. Geraghty B, Graham EA, Logan B, et al. Methadone levels in breast milk. J Hum Lact. 1997;13(3):227-230.
36. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, et al. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
37. Novick DM, Joseph H, Croxson TS, et al. Absence of antibody to human immunodeficiency virus in long-term, socially rehabilitated methadone maintenance patients. Arch Intern Med. 1990;150(1):97-99.
38. Gowing LR, Farrell M, Bornemann R, et al. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195.
39. Nurco DN, Ball JC, Shaffer JW, et al. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102.
40. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468.
41. Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747-753.
42. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
43. Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: a review. Experimental and Clinical Psychopharmacology. 1995;3(4):477-489.
44. U.S. Food and Drug Administration. FDA approves first buprenorphine implant for treatment of opioid dependence. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm503719.htm. Published May 26, 2016. Accessed July 18, 2016.
45. The National Alliance of Advocates for Buprenorphine Treatment. https://www.naabt.org/index.cfm. Accessed July 18, 2016.
46. Buprenorphine: an alternative to methadone. Med Lett Drugs Ther. 2003;45(1150):13-15.
47. Sigmon SC, Dunn KE, Saulsgiver K, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013;70(12):1347-1354.
48. Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825-834.
49. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
50. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1-2):69-78.
51. Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727-1740.
52. Robinson GM, Dukes PD, Robinson BJ, et al. The misuse of buprenorphine and a buprenorphine-naloxone combination in Wellington, New Zealand. Drug Alcohol Depend. 1993;33(1):81-86.
53. Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958.
Communities across the United States have experienced a near-epidemic of opioid abuse. Deaths from opioid overdose have doubled since 2000 and increased 14% from 2013 to 2014.1 Treatment strategies for opioid use disorder (OUD) target individual well-being with the goal of preventing relapse. Most treatment approaches help patients gain self-confidence and have been described by patients as “giving them their life back.”
Broadly, the major phases of treatment for OUD are similar to those for other substance use disorders, and involve acute detoxification followed by long-term maintenance of sobriety. There are 3 main phases of treatment for OUD:
- treatment engagement
- stabilization and harm reduction
- sustained abstinence.
Long-term maintenance of sobriety in OUD could involve medications that are FDA-approved for this indication: methadone, buprenorphine, and naltrexone. Psychosocial interventions can be used individually and in combination with pharmacotherapy. Because OUD is a chronic disorder typically characterized by intermittent relapse, patients could move back and forth between the different phases of treatment.
In this article, we highlight the medication and non-medication treatment options for the long-term management of OUD.
Defining abuseExogenous opioids are synthetic substances used for their analgesic and morphine-like properties. Opioids are indicated for the treatment of certain pain conditions and exist in varying potencies and delivery systems, which are tailored for specific types of pain.2 Opioids work through activity at opioid receptors found in the brain, spinal cord, gut, and other organs. Agonism of specific opioid receptors results in a decreased perception of pain and contributes to their abuse potential.
Because of their abuse potential, prescription of opioids is governed by the Controlled Substances Act3 of the United States. Despite these regulations, approximately 4.5% of U.S. adults from a nationally representative sample were found to be misusing prescription opioids.4 Another study used data from the National Survey on Drug Use and Health and showed an increasing prevalence of OUD involving prescription drugs and resulting in increased mortality.5 The mortality rate from prescription opioids was found to be higher than for all other illicit drugs combined in 2013.6 Recently, Congress passed the Comprehensive Addiction and Recovery Act, which is intended to address the opioid crisis by expanding access to treatments for opioid overdose and addiction treatment services.7
The term “opioid use disorder” is found in DSM-58 and replaces the previous diagnoses of opioid abuse and dependence in DSM-IV-TR.9 OUD is characterized by a strong desire to continue using opioids despite problems associated with their use. Patients with OUD often experience cravings for opioids, tolerance, repeated failures at cutting down or limiting use, and decreased involvement in social activities.
Maladaptive use of opioids can result in physiologic dependence and lead to withdrawal symptoms, including anxiety, drug craving, insomnia, rhinorrhea, lacrimation, diarrhea, and piloerection. However, physiologic dependence might not necessarily lead to development of OUD, which can be diagnosed when enough other diagnostic criteria present in the absence of physiologic dependence.
Misuse of prescription opioids is more prevalent among patients who meet criteria for other substance use disorders than among patients who do not.10 Misuse of prescription opioids often results in greater health care utilization, including the need for emergency services, hospitalization, and detoxification.
Assessing for OUDThe Addiction Severity Index was designed to classify and monitor misuse of opioids. The Index has poor sensitivity; it detects recent opioid use but fails to differentiate patients using prescribed opioids from those who are abusing opioids.11 By comparison, the Current Opioid Misuse Measure (COMM) was developed to monitor opioid use in chemical dependency treatment settings.12 The COMM has reliable internal consistency13 and validity and can be used to assess use of opioids outside of routine medical care. To gauge the severity of withdrawal symptoms, the Objective Opioid Withdrawal Scale or Clinical Opiate Withdrawal Scale14 can be used. Table 1 summarizes some standardized tools used to assess OUD.
Neurobiological considerationsOpioids work through activity at mu, kappa, and delta opioid receptors. These receptors are present in both the peripheral and the central nervous system. Exogenous opioids work through activity at G protein-coupled receptors and by activating specific neurotransmitter systems. The effect of a given opioid drug is dependent on the type and location of receptors it modulates and can range from CNS depression to euphoria.
For example, mu receptor activation produces sedation, euphoria, or analgesia depending upon the location, frequency, and duration of receptor occupancy. Activation of CNS mu receptors can cause miosis and respiratory depression, whereas mu receptor activation in the peripheral nervous system can cause constipation and cough suppression. Mu receptor stimulation through various G-proteins triggers the second messenger cascade, generating enzymes such as cyclic adenosine monophosphate.
Clinical considerationsTreating OUD is challenging because of the ease with which patients can obtain opioids and because sometimes OUD occurs iatrogenically. Engaging patients in treatment is an important step in recovery, but it does not necessarily lead to reduction in opioid use. The engagement stage can involve outreach workers to encourage further treatment. Developing a therapeutic alliance and appropriately incentivizing patients also promotes entry into treatment. Motivational interviewing is used often in substance use treatment programs and can help engage patients in treatment and evaluate their willingness to change problematic behaviors.
Managing acute withdrawal symptoms. Withdrawal symptoms usually are not life threatening, but can be in the context of other medical conditions, such as autonomic instability, hypertension, cardiovascular disease, and dehydration. Withdrawal symptoms also can be life-threatening during in utero exposure to a fetus. Pharmacotherapeutic options to treat opioid withdrawal symptoms include long-acting opioids, such as methadone and buprenorphine,15 which can be administered in an ambulatory setting. The combination of buprenorphine and naloxone also can be used to treat opioid withdrawal symptoms.
The alpha-2 agonist16 clonidine, although not FDA-approved for OUD or opioid withdrawal, could be used to shorten the duration of withdrawal symptoms. Clonidine also decreases methadone withdrawal and can be combined with naloxone to target naloxone-induced opioid withdrawal symptoms.17,18 Nalbuphine and butorphanol should be avoided during opioid withdrawal because they antagonize opioid receptors and can precipitate withdrawal symptoms.19,20
Maintenance phase involves long-term stabilization and relapse prevention. Treatment options include medication and non-medication interventions.21
Non-pharmacologic treatment options,22 principally psychosocial interventions, can be used on their own or in combination with medications for maintenance treatment of OUD. Psychosocial interventions include structured, professionally administered interventions such as cognitive-behavioral therapy (CBT), aversion therapy, and day-treatment programs. Interventions such as peer counseling and self-help groups also are considered psychosocial interventions, but do not require the same type of professional training.
Peer support groups such as Narcotics Anonymous (NA) help members achieve and maintain sobriety and often focus on a traditional 12-step format or on the more recent Matrix Model,23,24 which is an intensive outpatient treatment program based on components of relapse prevention, motivational interviewing, CBT, and psychoeducation.
In these peer support models, group members discuss patterns of substance use and help one another recognize and overcome problematic behaviors. Groups may vary in terms of their specific approach. For example, NA encourages group members to focus on addiction itself while Methadone Anonymous prefers participants also discuss pharmacologic treatment experiences. Additional services for finding housing and assisting with job placement are also part of some relapse prevention strategies.
Although studies on the use of abstinence-based treatments are limited, abstinence-based therapy is an option for patients wishing to undergo chemical dependency treatment without taking prescription medications to address cravings or withdrawal symptoms.25 However, abstinence-based treatments have been shown to be less effective in improving outcomes than medication-assisted treatment (MAT).26 MAT combines medications and behavioral therapies for treating substance use disorders.
Pharmacologically, OUD can be treated with opioid agonist and antagonist medications. As summarized in Tables 2-4,27-31 these medications differ based on their pharmacokinetic and pharmacodynamic profiles and intrinsic activity at mu opioid receptors. Opioid system agonists, such as methadone and buprenorphine, decrease cravings by mimicking the activity of exogenous opioids. The opioid antagonists naloxone and naltrexone reinforce abstinence by inhibiting the euphoric effects associated with opioid use. The medication of choice for a given patient depends on:
- treatment adherence
- clinical setting
- degree of withdrawal symptoms
- motivation.26
If a patient is actively seeking abstinence from opioids, either agonist or antagonist treatment can be used. In cases where a patient is not seeking abstinence, then preference should be given to opioid agonists to prevent overdose.26
Evidence suggests MAT can improve outcomes with OUD when compared with abstinence treatment alone. Several randomized, controlled, trials showed methadone and buprenorphine were more effective at treating OUD compared with treatment without medication. To date, 3 medications have been FDA-approved for treating OUD: methadone, buprenorphine, and naltrexone.26 All 3 medications differ in their pharmacokinetic and pharmacodynamic profiles and intrinsic activities at central mu-opioid receptor, as summarized in Tables 2-4.27-31
MethadoneMethadone reduces the euphoric effects of opioid use because it binds to and blocks opioid receptors. Methadone is an opioid replacement strategy; higher dosages are used for maintenance treatment to prevent additional dosages of opioids from causing euphoria. Methadone typically is administered once daily. However, in certain circumstances, such as rapid metabolism or pregnancy, it can be given as a twice-daily dosing regimen. Specific ABCB1 variants and DRD2 genetic polymorphisms (simultaneous occurrence of ≥2 genetically determined phenotypes) might determine the dosage requirements of methadone.32
Methadone during pregnancy. Methadone is the treatment of choice for opioid-dependent women during pregnancy33 and is listed as pregnancy category C because it can result in physiologic dependence of the newborn, although there are no documented controlled studies in humans to assess this risk. Methadone can be used while breast-feeding as long as patients are HIV-negative and not abusing other drugs.34,35 Because the methadone concentration in breast milk generally is low, the medication can be administered to nursing mothers after a careful consideration of risks and benefits.
Methadone administration. There are stringent eligibility criteria for methadone administration; not all physicians are authorized to prescribe methadone. Its use is federally regulated and only licensed treatment programs and licensed inpatient detoxification units can prescribe and dispense methadone in controlled settings and under the direct supervision of clinical personnel (Table 5).36 Patients meeting eligibility criteria can attend a specialized methadone clinic.
One of the challenges when using methadone for long-term management of OUD is tapering the dosage and attempting to discontinue the medication. Discontinuation of methadone leads to withdrawal symptoms and requires a carefully tailored tapering schedule. The literature on methadone tapering is limited. Tapering schedules could differ from practice to practice and, in many cases, are highly individualized based on the need and response of specific patients.
Dosage reduction schedules can last from 2 to 3 weeks to 6 months. Studies indicate rapid reduction worsens treatment outcomes and protracted tapering is associated with better outcomes. A suggested tapering schedule could involve decreasing the dosage by 20% to 25% until reaching a dosage of 30 mg/d, then decreasing by 5 mg/d every 3 to 5 days until reaching a dosage of 10 mg/d, before finally decreasing by 2.5 mg/d every 3 to 5 days.
Some randomized trials have shown better outcomes with long-term treatment. The goal of many programs is transitioning from maintenance treatment to abstinence. However, programs targeting maintenance rather than abstinence have been shown to be more effective.
The FDA has no defined limits for treatment duration with either methadone or buprenorphine. Therefore, the decision to taper or discontinue either medication should be made carefully case by case, using sound clinical judgment. Studies show that methadone treatment could reduce the spread of HIV,37,38 decrease criminal behaviors,39 and reduce overall mortality rates.40 A follow-up study comparing individuals randomly assigned to receive methadone or buprenorphine for OUD showed reduced risk of mortality overall40 in both groups.
Adverse events reported during treatment with methadone include decreased libido, erectile dysfunction, constipation, drowsiness, QTc prolongation, and torsade de pointes.41 Therefore, the FDA recommends obtaining a detailed medical history and baseline electrocardiogram (ECG), with a repeat ECG within the first month of treatment and then annually. Informing patients about the possibility of arrhythmias is part of the informed consent process before starting methadone.
Clinicians also should be vigilant when using methadone in combination with other medications that can prolong the QTc interval (eg, some antipsychotics). Methadone has a greater risk of fatal overdose then buprenorphine. A large-scale study of >16,000 patients reported a 4-fold increase in mortality resulting from methadone overdose compared with buprenorphine.42
BuprenorphineBuprenorphine is a partial opioid agonist at the mu opioid receptor. A full opioid agonist binds and fully activates the opioid receptors; an antagonist blocks the same. An opioid receptor partial agonist partially activates the receptor. Therefore, an opioid system partial agonist is a functional antagonist and, at lower dosages, has weak agonist effects; at higher dosages, a partial agonist antagonizes other endogenous and exogenous opioids that compete for binding at the same receptor.43 Because of the partial agonist effect, buprenorphine could result in less physical dependence and less withdrawal symptoms.
Administration. In contrast to methadone, buprenorphine can be prescribed by physicians for long-term management of OUD in the United States. Buprenorphine is available in 2 formulations: a sublingual form for daily use and a long-acting form that causes less withdrawal symptoms and cravings. In May 2016 the FDA approved the first buprenorphine implant for use in opioid dependence.44,45
To prevent withdrawal symptoms, a 24-hour period of opioid abstinence is recommended before starting buprenorphine or buprenorphine/naloxone treatment.46 Although lacking empirical evidence, catechol-O-methyltransferase (COMT) inhibitors, such as entacapone, have an anti-craving affect and are used by some clinicians to improve adherence with buprenorphine. This is because of their ability to balance dopamine, which is central to the reward pathway responsible for cravings. Although use of COMT inhibitors might make sense intuitively, such use is off-label and should be based on clinical judgment and a review of the available literature. A study showed that tapering buprenorphine for 4 weeks in combination with naltrexone improved the abstinence rate.47
Adverse effects. Some of the adverse events reported during treatment with buprenorphine include fever, back pain, nausea, cough, sedation, difficulty with urination, and constipation. Respiratory depression is a less common effect of buprenorphine, compared with full opioid agonists, because of the medication’s mechanism of action as a partial agonist.48 As a result, buprenorphine has been shown to have a lower risk of fatal overdose than methadone.49 Studies have shown buprenorphine to be more likely than methadone to reduce neonatal abstinence syndromes.50
NaltrexoneNaltrexone is an opioid antagonist and is an option to promote relapse prevention. Because of its antagonist properties, naltrexone treatment should always start after opioid detoxification because it can potentiate immediate withdrawal symptoms. Naltrexone is available in oral and long-acting formulations, the latter of which may be considered in patients who have difficulty with adherence.37
Oral naltrexone is taken as a single 50 mg-tablet once daily, whereas dosing for long-acting naltrexone in injectable and implantable formulations varies. These long-acting naltrexone formulations typically are administered monthly. Some of the adverse events reported during treatment with naltrexone are nausea, liver damage, and injection site pain.51
Buprenorphine/naloxoneBecause of buprenorphine’s agonist effects, it has a relatively high abuse potential compared with other opioids.52 Naloxone, on the other hand, is an opioid antagonist and is poorly absorbed when given orally and is associated with withdrawal symptoms if used intravenously. Therefore, naloxone is added to buprenorphine to decrease the likelihood of abuse when both are used as a combination product.53
Buprenorphine is combined with naloxone in a ratio of 4:1. Induction begins by using a 2 mg/0.5 mg tablet with dosage titration until symptoms abate. A combination of buprenorphine and naloxone also is available in film and tablet formulations. Patients must abstain from other opioids for at least 24 hours before initiating buprenorphine/naloxone treatment to prevent the precipitation of withdrawal symptoms.
Communities across the United States have experienced a near-epidemic of opioid abuse. Deaths from opioid overdose have doubled since 2000 and increased 14% from 2013 to 2014.1 Treatment strategies for opioid use disorder (OUD) target individual well-being with the goal of preventing relapse. Most treatment approaches help patients gain self-confidence and have been described by patients as “giving them their life back.”
Broadly, the major phases of treatment for OUD are similar to those for other substance use disorders, and involve acute detoxification followed by long-term maintenance of sobriety. There are 3 main phases of treatment for OUD:
- treatment engagement
- stabilization and harm reduction
- sustained abstinence.
Long-term maintenance of sobriety in OUD could involve medications that are FDA-approved for this indication: methadone, buprenorphine, and naltrexone. Psychosocial interventions can be used individually and in combination with pharmacotherapy. Because OUD is a chronic disorder typically characterized by intermittent relapse, patients could move back and forth between the different phases of treatment.
In this article, we highlight the medication and non-medication treatment options for the long-term management of OUD.
Defining abuseExogenous opioids are synthetic substances used for their analgesic and morphine-like properties. Opioids are indicated for the treatment of certain pain conditions and exist in varying potencies and delivery systems, which are tailored for specific types of pain.2 Opioids work through activity at opioid receptors found in the brain, spinal cord, gut, and other organs. Agonism of specific opioid receptors results in a decreased perception of pain and contributes to their abuse potential.
Because of their abuse potential, prescription of opioids is governed by the Controlled Substances Act3 of the United States. Despite these regulations, approximately 4.5% of U.S. adults from a nationally representative sample were found to be misusing prescription opioids.4 Another study used data from the National Survey on Drug Use and Health and showed an increasing prevalence of OUD involving prescription drugs and resulting in increased mortality.5 The mortality rate from prescription opioids was found to be higher than for all other illicit drugs combined in 2013.6 Recently, Congress passed the Comprehensive Addiction and Recovery Act, which is intended to address the opioid crisis by expanding access to treatments for opioid overdose and addiction treatment services.7
The term “opioid use disorder” is found in DSM-58 and replaces the previous diagnoses of opioid abuse and dependence in DSM-IV-TR.9 OUD is characterized by a strong desire to continue using opioids despite problems associated with their use. Patients with OUD often experience cravings for opioids, tolerance, repeated failures at cutting down or limiting use, and decreased involvement in social activities.
Maladaptive use of opioids can result in physiologic dependence and lead to withdrawal symptoms, including anxiety, drug craving, insomnia, rhinorrhea, lacrimation, diarrhea, and piloerection. However, physiologic dependence might not necessarily lead to development of OUD, which can be diagnosed when enough other diagnostic criteria present in the absence of physiologic dependence.
Misuse of prescription opioids is more prevalent among patients who meet criteria for other substance use disorders than among patients who do not.10 Misuse of prescription opioids often results in greater health care utilization, including the need for emergency services, hospitalization, and detoxification.
Assessing for OUDThe Addiction Severity Index was designed to classify and monitor misuse of opioids. The Index has poor sensitivity; it detects recent opioid use but fails to differentiate patients using prescribed opioids from those who are abusing opioids.11 By comparison, the Current Opioid Misuse Measure (COMM) was developed to monitor opioid use in chemical dependency treatment settings.12 The COMM has reliable internal consistency13 and validity and can be used to assess use of opioids outside of routine medical care. To gauge the severity of withdrawal symptoms, the Objective Opioid Withdrawal Scale or Clinical Opiate Withdrawal Scale14 can be used. Table 1 summarizes some standardized tools used to assess OUD.
Neurobiological considerationsOpioids work through activity at mu, kappa, and delta opioid receptors. These receptors are present in both the peripheral and the central nervous system. Exogenous opioids work through activity at G protein-coupled receptors and by activating specific neurotransmitter systems. The effect of a given opioid drug is dependent on the type and location of receptors it modulates and can range from CNS depression to euphoria.
For example, mu receptor activation produces sedation, euphoria, or analgesia depending upon the location, frequency, and duration of receptor occupancy. Activation of CNS mu receptors can cause miosis and respiratory depression, whereas mu receptor activation in the peripheral nervous system can cause constipation and cough suppression. Mu receptor stimulation through various G-proteins triggers the second messenger cascade, generating enzymes such as cyclic adenosine monophosphate.
Clinical considerationsTreating OUD is challenging because of the ease with which patients can obtain opioids and because sometimes OUD occurs iatrogenically. Engaging patients in treatment is an important step in recovery, but it does not necessarily lead to reduction in opioid use. The engagement stage can involve outreach workers to encourage further treatment. Developing a therapeutic alliance and appropriately incentivizing patients also promotes entry into treatment. Motivational interviewing is used often in substance use treatment programs and can help engage patients in treatment and evaluate their willingness to change problematic behaviors.
Managing acute withdrawal symptoms. Withdrawal symptoms usually are not life threatening, but can be in the context of other medical conditions, such as autonomic instability, hypertension, cardiovascular disease, and dehydration. Withdrawal symptoms also can be life-threatening during in utero exposure to a fetus. Pharmacotherapeutic options to treat opioid withdrawal symptoms include long-acting opioids, such as methadone and buprenorphine,15 which can be administered in an ambulatory setting. The combination of buprenorphine and naloxone also can be used to treat opioid withdrawal symptoms.
The alpha-2 agonist16 clonidine, although not FDA-approved for OUD or opioid withdrawal, could be used to shorten the duration of withdrawal symptoms. Clonidine also decreases methadone withdrawal and can be combined with naloxone to target naloxone-induced opioid withdrawal symptoms.17,18 Nalbuphine and butorphanol should be avoided during opioid withdrawal because they antagonize opioid receptors and can precipitate withdrawal symptoms.19,20
Maintenance phase involves long-term stabilization and relapse prevention. Treatment options include medication and non-medication interventions.21
Non-pharmacologic treatment options,22 principally psychosocial interventions, can be used on their own or in combination with medications for maintenance treatment of OUD. Psychosocial interventions include structured, professionally administered interventions such as cognitive-behavioral therapy (CBT), aversion therapy, and day-treatment programs. Interventions such as peer counseling and self-help groups also are considered psychosocial interventions, but do not require the same type of professional training.
Peer support groups such as Narcotics Anonymous (NA) help members achieve and maintain sobriety and often focus on a traditional 12-step format or on the more recent Matrix Model,23,24 which is an intensive outpatient treatment program based on components of relapse prevention, motivational interviewing, CBT, and psychoeducation.
In these peer support models, group members discuss patterns of substance use and help one another recognize and overcome problematic behaviors. Groups may vary in terms of their specific approach. For example, NA encourages group members to focus on addiction itself while Methadone Anonymous prefers participants also discuss pharmacologic treatment experiences. Additional services for finding housing and assisting with job placement are also part of some relapse prevention strategies.
Although studies on the use of abstinence-based treatments are limited, abstinence-based therapy is an option for patients wishing to undergo chemical dependency treatment without taking prescription medications to address cravings or withdrawal symptoms.25 However, abstinence-based treatments have been shown to be less effective in improving outcomes than medication-assisted treatment (MAT).26 MAT combines medications and behavioral therapies for treating substance use disorders.
Pharmacologically, OUD can be treated with opioid agonist and antagonist medications. As summarized in Tables 2-4,27-31 these medications differ based on their pharmacokinetic and pharmacodynamic profiles and intrinsic activity at mu opioid receptors. Opioid system agonists, such as methadone and buprenorphine, decrease cravings by mimicking the activity of exogenous opioids. The opioid antagonists naloxone and naltrexone reinforce abstinence by inhibiting the euphoric effects associated with opioid use. The medication of choice for a given patient depends on:
- treatment adherence
- clinical setting
- degree of withdrawal symptoms
- motivation.26
If a patient is actively seeking abstinence from opioids, either agonist or antagonist treatment can be used. In cases where a patient is not seeking abstinence, then preference should be given to opioid agonists to prevent overdose.26
Evidence suggests MAT can improve outcomes with OUD when compared with abstinence treatment alone. Several randomized, controlled, trials showed methadone and buprenorphine were more effective at treating OUD compared with treatment without medication. To date, 3 medications have been FDA-approved for treating OUD: methadone, buprenorphine, and naltrexone.26 All 3 medications differ in their pharmacokinetic and pharmacodynamic profiles and intrinsic activities at central mu-opioid receptor, as summarized in Tables 2-4.27-31
MethadoneMethadone reduces the euphoric effects of opioid use because it binds to and blocks opioid receptors. Methadone is an opioid replacement strategy; higher dosages are used for maintenance treatment to prevent additional dosages of opioids from causing euphoria. Methadone typically is administered once daily. However, in certain circumstances, such as rapid metabolism or pregnancy, it can be given as a twice-daily dosing regimen. Specific ABCB1 variants and DRD2 genetic polymorphisms (simultaneous occurrence of ≥2 genetically determined phenotypes) might determine the dosage requirements of methadone.32
Methadone during pregnancy. Methadone is the treatment of choice for opioid-dependent women during pregnancy33 and is listed as pregnancy category C because it can result in physiologic dependence of the newborn, although there are no documented controlled studies in humans to assess this risk. Methadone can be used while breast-feeding as long as patients are HIV-negative and not abusing other drugs.34,35 Because the methadone concentration in breast milk generally is low, the medication can be administered to nursing mothers after a careful consideration of risks and benefits.
Methadone administration. There are stringent eligibility criteria for methadone administration; not all physicians are authorized to prescribe methadone. Its use is federally regulated and only licensed treatment programs and licensed inpatient detoxification units can prescribe and dispense methadone in controlled settings and under the direct supervision of clinical personnel (Table 5).36 Patients meeting eligibility criteria can attend a specialized methadone clinic.
One of the challenges when using methadone for long-term management of OUD is tapering the dosage and attempting to discontinue the medication. Discontinuation of methadone leads to withdrawal symptoms and requires a carefully tailored tapering schedule. The literature on methadone tapering is limited. Tapering schedules could differ from practice to practice and, in many cases, are highly individualized based on the need and response of specific patients.
Dosage reduction schedules can last from 2 to 3 weeks to 6 months. Studies indicate rapid reduction worsens treatment outcomes and protracted tapering is associated with better outcomes. A suggested tapering schedule could involve decreasing the dosage by 20% to 25% until reaching a dosage of 30 mg/d, then decreasing by 5 mg/d every 3 to 5 days until reaching a dosage of 10 mg/d, before finally decreasing by 2.5 mg/d every 3 to 5 days.
Some randomized trials have shown better outcomes with long-term treatment. The goal of many programs is transitioning from maintenance treatment to abstinence. However, programs targeting maintenance rather than abstinence have been shown to be more effective.
The FDA has no defined limits for treatment duration with either methadone or buprenorphine. Therefore, the decision to taper or discontinue either medication should be made carefully case by case, using sound clinical judgment. Studies show that methadone treatment could reduce the spread of HIV,37,38 decrease criminal behaviors,39 and reduce overall mortality rates.40 A follow-up study comparing individuals randomly assigned to receive methadone or buprenorphine for OUD showed reduced risk of mortality overall40 in both groups.
Adverse events reported during treatment with methadone include decreased libido, erectile dysfunction, constipation, drowsiness, QTc prolongation, and torsade de pointes.41 Therefore, the FDA recommends obtaining a detailed medical history and baseline electrocardiogram (ECG), with a repeat ECG within the first month of treatment and then annually. Informing patients about the possibility of arrhythmias is part of the informed consent process before starting methadone.
Clinicians also should be vigilant when using methadone in combination with other medications that can prolong the QTc interval (eg, some antipsychotics). Methadone has a greater risk of fatal overdose then buprenorphine. A large-scale study of >16,000 patients reported a 4-fold increase in mortality resulting from methadone overdose compared with buprenorphine.42
BuprenorphineBuprenorphine is a partial opioid agonist at the mu opioid receptor. A full opioid agonist binds and fully activates the opioid receptors; an antagonist blocks the same. An opioid receptor partial agonist partially activates the receptor. Therefore, an opioid system partial agonist is a functional antagonist and, at lower dosages, has weak agonist effects; at higher dosages, a partial agonist antagonizes other endogenous and exogenous opioids that compete for binding at the same receptor.43 Because of the partial agonist effect, buprenorphine could result in less physical dependence and less withdrawal symptoms.
Administration. In contrast to methadone, buprenorphine can be prescribed by physicians for long-term management of OUD in the United States. Buprenorphine is available in 2 formulations: a sublingual form for daily use and a long-acting form that causes less withdrawal symptoms and cravings. In May 2016 the FDA approved the first buprenorphine implant for use in opioid dependence.44,45
To prevent withdrawal symptoms, a 24-hour period of opioid abstinence is recommended before starting buprenorphine or buprenorphine/naloxone treatment.46 Although lacking empirical evidence, catechol-O-methyltransferase (COMT) inhibitors, such as entacapone, have an anti-craving affect and are used by some clinicians to improve adherence with buprenorphine. This is because of their ability to balance dopamine, which is central to the reward pathway responsible for cravings. Although use of COMT inhibitors might make sense intuitively, such use is off-label and should be based on clinical judgment and a review of the available literature. A study showed that tapering buprenorphine for 4 weeks in combination with naltrexone improved the abstinence rate.47
Adverse effects. Some of the adverse events reported during treatment with buprenorphine include fever, back pain, nausea, cough, sedation, difficulty with urination, and constipation. Respiratory depression is a less common effect of buprenorphine, compared with full opioid agonists, because of the medication’s mechanism of action as a partial agonist.48 As a result, buprenorphine has been shown to have a lower risk of fatal overdose than methadone.49 Studies have shown buprenorphine to be more likely than methadone to reduce neonatal abstinence syndromes.50
NaltrexoneNaltrexone is an opioid antagonist and is an option to promote relapse prevention. Because of its antagonist properties, naltrexone treatment should always start after opioid detoxification because it can potentiate immediate withdrawal symptoms. Naltrexone is available in oral and long-acting formulations, the latter of which may be considered in patients who have difficulty with adherence.37
Oral naltrexone is taken as a single 50 mg-tablet once daily, whereas dosing for long-acting naltrexone in injectable and implantable formulations varies. These long-acting naltrexone formulations typically are administered monthly. Some of the adverse events reported during treatment with naltrexone are nausea, liver damage, and injection site pain.51
Buprenorphine/naloxoneBecause of buprenorphine’s agonist effects, it has a relatively high abuse potential compared with other opioids.52 Naloxone, on the other hand, is an opioid antagonist and is poorly absorbed when given orally and is associated with withdrawal symptoms if used intravenously. Therefore, naloxone is added to buprenorphine to decrease the likelihood of abuse when both are used as a combination product.53
Buprenorphine is combined with naloxone in a ratio of 4:1. Induction begins by using a 2 mg/0.5 mg tablet with dosage titration until symptoms abate. A combination of buprenorphine and naloxone also is available in film and tablet formulations. Patients must abstain from other opioids for at least 24 hours before initiating buprenorphine/naloxone treatment to prevent the precipitation of withdrawal symptoms.
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
2. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695-1700.
3. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
4. Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94(1-3):38-47.
5. Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015;314(14):1468-1478.
6. Centers for Disease Control and Prevention. National Center for Health Statistics, 2014. Multiple cause of death data. http://wonder.cdc.gov/mcd.html.
7. Twachtman G. Congress sends opioid legislation to the President. Clinical Psychiatry News. http://www.clinicalpsychiatrynews.com/?id=2407&tx_ttnews[tt_news]=524025&cHash=e93d5d1f86d20e53d3e2d8b07e9562b2. Published July 15, 2016. Accessed July 18, 2016.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
10. McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav. 2008;33(10):1297-1305.
11. Butler SF, Villapiano A, Malinow A. The effect of computer-mediated administration on self-disclosure of problems on the Addiction Severity Index. J Addict Med. 2009;3(4):194-203.
12. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152(2):397-402.
13. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26(9):770-776.
14. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259.
15. Fiellin DA, O’Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347(11):817-823.
16. Gowing LR, Farrell M, Ali RL, et al. α2‐Adrenergic agonists in opioid withdrawal. Addiction. 2002;97(1):49-58.
17. Loimer N, Hofmann P, Chaudhry H. Ultrashort noninvasive opiate detoxification. Am J Psychiatry. 1993;150(5):839.
18. Charney DS, Sternberg DE, Kleber HD, et al. The clinical use of clonidine in abrupt withdrawal from methadone. Effects on blood pressure and specific signs and symptoms. Arch Gen Psychiatry. 1981;38(11):1273-1277.
19. Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1989;248(3):929-937.
20. Preston KL, Bigelow GE, Liebson IA. Discrimination of butorphanol and nalbuphine in opioid-dependent humans. Pharmacol Biochem Behav. 1990;37(3):511-522.
21. Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280(22):1936-1943.
22. Amato L, Minozzi S, Davoli, M, et al. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4.
23. Obert JL, McCann MJ, Marinelli-Casey P, et al. The matrix model of outpatient stimulant abuse treatment: history and description. J Psychoactive Drugs. 2000;32(2):157-164.
24. Mayet S, Farrell M, Ferri M, et al. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2005:CD004330.
25. McAuliffe WE. A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. J Psychoactive Drugs. 1990;22(2):197-209.
26. Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
27. Gibson AE, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug Alcohol Rev. 2007;26:405-410.
28. Clark L, Haram E, Johnson K, et al. Getting started with medication-assisted treatment with lessons from advancing recovery. Madison, WI: University of Wisconsin-Madison; 2010.
29. U.S. Food and Drug Administration. Vivitrol (naltrexone for extended-release injectable suspension): NDA 21-897C—Briefing document/background package. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharmacologic DrugsAdvisoryCommittee/UCM225664.pdf. Published September 16, 2010. Accessed July 11, 2016.
30. Providers Clinical Support System. PCSS Guidance. Buprenorphine induction. http://pcssmat.org/wp-content/uploads/2014/02/PCSS-MATGuidanceBuprenorphineInduction.Casadonte.pdf. Updated November 27, 2013. Accessed July 18, 2016.
31. An introduction to extended-release injectable naltrexone for the treatment of people with opioid dependence. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. HHS Publication No. (SMA) 12-4682.
32. Doehring A, von Hentig N, Graff J, et al. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19(6):407-414.
33. Mitchell JL. Pregnant, substance-abusing women: treatment improvement protocol (TIP) Series 2. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1993. DHHS Publication No. (SMA) 95-3056.
34. McCarthy JJ, Posey BL. Methadone levels in human milk. J Hum Lact. 2000;16(2):115-120.
35. Geraghty B, Graham EA, Logan B, et al. Methadone levels in breast milk. J Hum Lact. 1997;13(3):227-230.
36. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, et al. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
37. Novick DM, Joseph H, Croxson TS, et al. Absence of antibody to human immunodeficiency virus in long-term, socially rehabilitated methadone maintenance patients. Arch Intern Med. 1990;150(1):97-99.
38. Gowing LR, Farrell M, Bornemann R, et al. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195.
39. Nurco DN, Ball JC, Shaffer JW, et al. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102.
40. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468.
41. Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747-753.
42. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
43. Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: a review. Experimental and Clinical Psychopharmacology. 1995;3(4):477-489.
44. U.S. Food and Drug Administration. FDA approves first buprenorphine implant for treatment of opioid dependence. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm503719.htm. Published May 26, 2016. Accessed July 18, 2016.
45. The National Alliance of Advocates for Buprenorphine Treatment. https://www.naabt.org/index.cfm. Accessed July 18, 2016.
46. Buprenorphine: an alternative to methadone. Med Lett Drugs Ther. 2003;45(1150):13-15.
47. Sigmon SC, Dunn KE, Saulsgiver K, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013;70(12):1347-1354.
48. Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825-834.
49. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
50. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1-2):69-78.
51. Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727-1740.
52. Robinson GM, Dukes PD, Robinson BJ, et al. The misuse of buprenorphine and a buprenorphine-naloxone combination in Wellington, New Zealand. Drug Alcohol Depend. 1993;33(1):81-86.
53. Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958.
1. Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
2. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695-1700.
3. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
4. Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94(1-3):38-47.
5. Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015;314(14):1468-1478.
6. Centers for Disease Control and Prevention. National Center for Health Statistics, 2014. Multiple cause of death data. http://wonder.cdc.gov/mcd.html.
7. Twachtman G. Congress sends opioid legislation to the President. Clinical Psychiatry News. http://www.clinicalpsychiatrynews.com/?id=2407&tx_ttnews[tt_news]=524025&cHash=e93d5d1f86d20e53d3e2d8b07e9562b2. Published July 15, 2016. Accessed July 18, 2016.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
10. McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav. 2008;33(10):1297-1305.
11. Butler SF, Villapiano A, Malinow A. The effect of computer-mediated administration on self-disclosure of problems on the Addiction Severity Index. J Addict Med. 2009;3(4):194-203.
12. Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152(2):397-402.
13. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26(9):770-776.
14. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259.
15. Fiellin DA, O’Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347(11):817-823.
16. Gowing LR, Farrell M, Ali RL, et al. α2‐Adrenergic agonists in opioid withdrawal. Addiction. 2002;97(1):49-58.
17. Loimer N, Hofmann P, Chaudhry H. Ultrashort noninvasive opiate detoxification. Am J Psychiatry. 1993;150(5):839.
18. Charney DS, Sternberg DE, Kleber HD, et al. The clinical use of clonidine in abrupt withdrawal from methadone. Effects on blood pressure and specific signs and symptoms. Arch Gen Psychiatry. 1981;38(11):1273-1277.
19. Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1989;248(3):929-937.
20. Preston KL, Bigelow GE, Liebson IA. Discrimination of butorphanol and nalbuphine in opioid-dependent humans. Pharmacol Biochem Behav. 1990;37(3):511-522.
21. Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280(22):1936-1943.
22. Amato L, Minozzi S, Davoli, M, et al. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4.
23. Obert JL, McCann MJ, Marinelli-Casey P, et al. The matrix model of outpatient stimulant abuse treatment: history and description. J Psychoactive Drugs. 2000;32(2):157-164.
24. Mayet S, Farrell M, Ferri M, et al. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2005:CD004330.
25. McAuliffe WE. A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. J Psychoactive Drugs. 1990;22(2):197-209.
26. Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
27. Gibson AE, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug Alcohol Rev. 2007;26:405-410.
28. Clark L, Haram E, Johnson K, et al. Getting started with medication-assisted treatment with lessons from advancing recovery. Madison, WI: University of Wisconsin-Madison; 2010.
29. U.S. Food and Drug Administration. Vivitrol (naltrexone for extended-release injectable suspension): NDA 21-897C—Briefing document/background package. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharmacologic DrugsAdvisoryCommittee/UCM225664.pdf. Published September 16, 2010. Accessed July 11, 2016.
30. Providers Clinical Support System. PCSS Guidance. Buprenorphine induction. http://pcssmat.org/wp-content/uploads/2014/02/PCSS-MATGuidanceBuprenorphineInduction.Casadonte.pdf. Updated November 27, 2013. Accessed July 18, 2016.
31. An introduction to extended-release injectable naltrexone for the treatment of people with opioid dependence. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. HHS Publication No. (SMA) 12-4682.
32. Doehring A, von Hentig N, Graff J, et al. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19(6):407-414.
33. Mitchell JL. Pregnant, substance-abusing women: treatment improvement protocol (TIP) Series 2. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1993. DHHS Publication No. (SMA) 95-3056.
34. McCarthy JJ, Posey BL. Methadone levels in human milk. J Hum Lact. 2000;16(2):115-120.
35. Geraghty B, Graham EA, Logan B, et al. Methadone levels in breast milk. J Hum Lact. 1997;13(3):227-230.
36. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, et al. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
37. Novick DM, Joseph H, Croxson TS, et al. Absence of antibody to human immunodeficiency virus in long-term, socially rehabilitated methadone maintenance patients. Arch Intern Med. 1990;150(1):97-99.
38. Gowing LR, Farrell M, Bornemann R, et al. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195.
39. Nurco DN, Ball JC, Shaffer JW, et al. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102.
40. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468.
41. Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747-753.
42. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
43. Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: a review. Experimental and Clinical Psychopharmacology. 1995;3(4):477-489.
44. U.S. Food and Drug Administration. FDA approves first buprenorphine implant for treatment of opioid dependence. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm503719.htm. Published May 26, 2016. Accessed July 18, 2016.
45. The National Alliance of Advocates for Buprenorphine Treatment. https://www.naabt.org/index.cfm. Accessed July 18, 2016.
46. Buprenorphine: an alternative to methadone. Med Lett Drugs Ther. 2003;45(1150):13-15.
47. Sigmon SC, Dunn KE, Saulsgiver K, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013;70(12):1347-1354.
48. Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825-834.
49. Bell JR, Butler B, Lawrance A, et al. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73-77.
50. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1-2):69-78.
51. Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727-1740.
52. Robinson GM, Dukes PD, Robinson BJ, et al. The misuse of buprenorphine and a buprenorphine-naloxone combination in Wellington, New Zealand. Drug Alcohol Depend. 1993;33(1):81-86.
53. Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958.
Fatigue after depression responds to therapy. What are the next steps?
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.