User login
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.
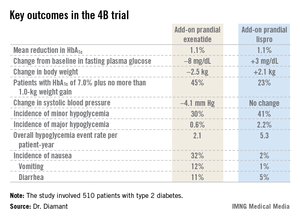
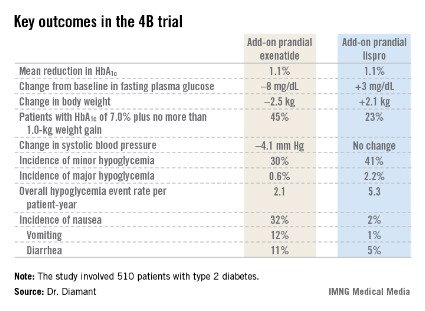
The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.

The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.

The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: Patients with inadequately controlled type 2 diabetes, despite their being on metformin and optimized basal insulin, had better outcomes in multiple domains if they received add-on premeal exenatide twice daily than with add-on prandial insulin lispro three times daily.
Data Source: A phase IIIb, open-label, prospective randomized trial involving 510 type 2 diabetic patients with inadequate glycemic control.
Disclosures: The trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.