User login
American Diabetes Association (ADA): Annual Scientific Sessions
Fibroblast growth factor 21 promising in treating metabolic disorders
CHICAGO – Fibroblast growth factor 21 shows early promise as a novel therapy for a variety of metabolic disorders closely associated with type 2 diabetes.
A first-in-humans, proof-of-concept randomized trial involving 38 patients with type 2 diabetes showed that a pharmaceutically engineered variant of endogenous fibroblast growth factor 21 (FGF21) achieved "rapid and robust" improvements in lipid levels along with weight loss and reduced fasting insulin levels, Dr. Gregory A. Gaich reported at the annual scientific sessions of the American Diabetes Association.
"These results suggest that FGF21-based therapeutics may be useful for select metabolic disorders, including dyslipidemia and obesity. However, higher doses and longer periods of exposure may be required to really fully assess the potential of FGF21-based interventions in patients with type 2 diabetes," said Dr. Gaich of Eli Lilly, Indianapolis.
FGF21, discovered by Eli Lilly scientists, is a nonmitogenic member of the fibroblast growth factor family. It is involved in energy expenditure, induction of brown fat, free fatty acid oxidation, thermogenesis, and other physiologic processes. In primate and other animal studies, administration of exogenous FGF21 showed beneficial effects on lipids, body weight, and serum glucose, observations that paved the way for this randomized trial.
Participants in the 28-day, double-blind study were randomized to once-daily subcutaneous injection of the FGF21 variant, known for now as LY2405319, at 3, 10, or 20 mg or to placebo.
Subjects who received either of the two highest doses of the study drug showed 40%-50% reductions from baseline in serum triglycerides within 7 days, with maintenance of that benefit throughout the remaining 3 weeks of the trial. In contrast, reductions in LDL cholesterol occurred more gradually. By day 28, patients on the FGF21 compound at 10 or 20 mg/day showed 20%-30% reductions in LDL; however, their levels were still falling at that point, so the maximum LDL-lowering effect must await further study.
The levels of HDL cholesterol rose significantly by 15%-20% at all 3 doses of FGF21.
Body weight in the FGF21-treated patients fell by a mean of 1.7 kg. "We were delighted to see what we thought was a pretty encouraging weight loss for such a short study," Dr. Gaich commented.
Reduction in fasting plasma glucose showed a favorable dose-dependent trend that didn’t achieve statistical difference. Patients assigned to FGF21 at 20 mg/day had a 13-mg/dL greater drop over the course of the study than placebo-treated controls.
The reduction in fasting insulin levels with FGF21 was more encouraging. The effect was dose-dependent, with subjects in the 20 mg/day group exhibiting a highly significant 40% decrease from baseline.
Levels of two biomarkers followed serially in the study provide a hint as to FGF21’s mechanism of action. Levels of adiponectin, which is associated with increased insulin sensitivity, increased in dose-dependent fashion across all three groups of FGF21-treated patients and were nearly doubled at 20 mg/day compared to baseline. And levels of beta-hydroxybutyrate, a biomarker for lipid oxidation, showed across-the-board increases of 50%-90%.
No dose-dependent increase in the incidence of hypoglycemia was noted in the study. The chief safety concern was the occurrence of significant hypersensitivity reactions in two FGF21-treated patients, both in the 20 mg/day group. One patient developed a diffuse rash roughly halfway through the treatment period. The other experienced a more severe hypersensitivity reaction, including hypotension, on the second-to-last day of treatment.
Anti-LY2405319 antibodies arose in 80%-87% of patients on the drug at 10 and 20 mg/day. The clinical significance, if any, of these antibodies awaits further study. In this small initial study, there was no association between antibody production and any of the efficacy or safety parameters. Only one of the two patients with hypersensitivity reactions was antibody-positive, according to Dr. Gaich.
Dr. Gaich is an employee of Eli Lilly, which sponsored the study.
CHICAGO – Fibroblast growth factor 21 shows early promise as a novel therapy for a variety of metabolic disorders closely associated with type 2 diabetes.
A first-in-humans, proof-of-concept randomized trial involving 38 patients with type 2 diabetes showed that a pharmaceutically engineered variant of endogenous fibroblast growth factor 21 (FGF21) achieved "rapid and robust" improvements in lipid levels along with weight loss and reduced fasting insulin levels, Dr. Gregory A. Gaich reported at the annual scientific sessions of the American Diabetes Association.
"These results suggest that FGF21-based therapeutics may be useful for select metabolic disorders, including dyslipidemia and obesity. However, higher doses and longer periods of exposure may be required to really fully assess the potential of FGF21-based interventions in patients with type 2 diabetes," said Dr. Gaich of Eli Lilly, Indianapolis.
FGF21, discovered by Eli Lilly scientists, is a nonmitogenic member of the fibroblast growth factor family. It is involved in energy expenditure, induction of brown fat, free fatty acid oxidation, thermogenesis, and other physiologic processes. In primate and other animal studies, administration of exogenous FGF21 showed beneficial effects on lipids, body weight, and serum glucose, observations that paved the way for this randomized trial.
Participants in the 28-day, double-blind study were randomized to once-daily subcutaneous injection of the FGF21 variant, known for now as LY2405319, at 3, 10, or 20 mg or to placebo.
Subjects who received either of the two highest doses of the study drug showed 40%-50% reductions from baseline in serum triglycerides within 7 days, with maintenance of that benefit throughout the remaining 3 weeks of the trial. In contrast, reductions in LDL cholesterol occurred more gradually. By day 28, patients on the FGF21 compound at 10 or 20 mg/day showed 20%-30% reductions in LDL; however, their levels were still falling at that point, so the maximum LDL-lowering effect must await further study.
The levels of HDL cholesterol rose significantly by 15%-20% at all 3 doses of FGF21.
Body weight in the FGF21-treated patients fell by a mean of 1.7 kg. "We were delighted to see what we thought was a pretty encouraging weight loss for such a short study," Dr. Gaich commented.
Reduction in fasting plasma glucose showed a favorable dose-dependent trend that didn’t achieve statistical difference. Patients assigned to FGF21 at 20 mg/day had a 13-mg/dL greater drop over the course of the study than placebo-treated controls.
The reduction in fasting insulin levels with FGF21 was more encouraging. The effect was dose-dependent, with subjects in the 20 mg/day group exhibiting a highly significant 40% decrease from baseline.
Levels of two biomarkers followed serially in the study provide a hint as to FGF21’s mechanism of action. Levels of adiponectin, which is associated with increased insulin sensitivity, increased in dose-dependent fashion across all three groups of FGF21-treated patients and were nearly doubled at 20 mg/day compared to baseline. And levels of beta-hydroxybutyrate, a biomarker for lipid oxidation, showed across-the-board increases of 50%-90%.
No dose-dependent increase in the incidence of hypoglycemia was noted in the study. The chief safety concern was the occurrence of significant hypersensitivity reactions in two FGF21-treated patients, both in the 20 mg/day group. One patient developed a diffuse rash roughly halfway through the treatment period. The other experienced a more severe hypersensitivity reaction, including hypotension, on the second-to-last day of treatment.
Anti-LY2405319 antibodies arose in 80%-87% of patients on the drug at 10 and 20 mg/day. The clinical significance, if any, of these antibodies awaits further study. In this small initial study, there was no association between antibody production and any of the efficacy or safety parameters. Only one of the two patients with hypersensitivity reactions was antibody-positive, according to Dr. Gaich.
Dr. Gaich is an employee of Eli Lilly, which sponsored the study.
CHICAGO – Fibroblast growth factor 21 shows early promise as a novel therapy for a variety of metabolic disorders closely associated with type 2 diabetes.
A first-in-humans, proof-of-concept randomized trial involving 38 patients with type 2 diabetes showed that a pharmaceutically engineered variant of endogenous fibroblast growth factor 21 (FGF21) achieved "rapid and robust" improvements in lipid levels along with weight loss and reduced fasting insulin levels, Dr. Gregory A. Gaich reported at the annual scientific sessions of the American Diabetes Association.
"These results suggest that FGF21-based therapeutics may be useful for select metabolic disorders, including dyslipidemia and obesity. However, higher doses and longer periods of exposure may be required to really fully assess the potential of FGF21-based interventions in patients with type 2 diabetes," said Dr. Gaich of Eli Lilly, Indianapolis.
FGF21, discovered by Eli Lilly scientists, is a nonmitogenic member of the fibroblast growth factor family. It is involved in energy expenditure, induction of brown fat, free fatty acid oxidation, thermogenesis, and other physiologic processes. In primate and other animal studies, administration of exogenous FGF21 showed beneficial effects on lipids, body weight, and serum glucose, observations that paved the way for this randomized trial.
Participants in the 28-day, double-blind study were randomized to once-daily subcutaneous injection of the FGF21 variant, known for now as LY2405319, at 3, 10, or 20 mg or to placebo.
Subjects who received either of the two highest doses of the study drug showed 40%-50% reductions from baseline in serum triglycerides within 7 days, with maintenance of that benefit throughout the remaining 3 weeks of the trial. In contrast, reductions in LDL cholesterol occurred more gradually. By day 28, patients on the FGF21 compound at 10 or 20 mg/day showed 20%-30% reductions in LDL; however, their levels were still falling at that point, so the maximum LDL-lowering effect must await further study.
The levels of HDL cholesterol rose significantly by 15%-20% at all 3 doses of FGF21.
Body weight in the FGF21-treated patients fell by a mean of 1.7 kg. "We were delighted to see what we thought was a pretty encouraging weight loss for such a short study," Dr. Gaich commented.
Reduction in fasting plasma glucose showed a favorable dose-dependent trend that didn’t achieve statistical difference. Patients assigned to FGF21 at 20 mg/day had a 13-mg/dL greater drop over the course of the study than placebo-treated controls.
The reduction in fasting insulin levels with FGF21 was more encouraging. The effect was dose-dependent, with subjects in the 20 mg/day group exhibiting a highly significant 40% decrease from baseline.
Levels of two biomarkers followed serially in the study provide a hint as to FGF21’s mechanism of action. Levels of adiponectin, which is associated with increased insulin sensitivity, increased in dose-dependent fashion across all three groups of FGF21-treated patients and were nearly doubled at 20 mg/day compared to baseline. And levels of beta-hydroxybutyrate, a biomarker for lipid oxidation, showed across-the-board increases of 50%-90%.
No dose-dependent increase in the incidence of hypoglycemia was noted in the study. The chief safety concern was the occurrence of significant hypersensitivity reactions in two FGF21-treated patients, both in the 20 mg/day group. One patient developed a diffuse rash roughly halfway through the treatment period. The other experienced a more severe hypersensitivity reaction, including hypotension, on the second-to-last day of treatment.
Anti-LY2405319 antibodies arose in 80%-87% of patients on the drug at 10 and 20 mg/day. The clinical significance, if any, of these antibodies awaits further study. In this small initial study, there was no association between antibody production and any of the efficacy or safety parameters. Only one of the two patients with hypersensitivity reactions was antibody-positive, according to Dr. Gaich.
Dr. Gaich is an employee of Eli Lilly, which sponsored the study.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Patients with type 2 diabetes who received fibroblast growth factor 21 responded with 40%-50% reductions in serum triglycerides within 7 days, and these reduced levels were maintained throughout the remaining 3 weeks of the study.
Data source: A randomized, prospective, double-blind, first-in-humans pilot study in 38 patients with type 2 diabetes assigned to 28 days of once-daily subcutaneous injection of a synthetic fibroblast growth factor 21 variant or to placebo.
Disclosures: The study was sponsored by Eli Lilly. The presenter is a company employee.
Dapagliflozin explored in type 1 diabetes
CHICAGO – Even as dapagliflozin’s resubmitted application for marketing approval for treatment of type 2 diabetes is being scrutinized by the Food and Drug Administration, the drug is under study as a novel potential oral therapy for type 1 diabetes.
"For type 1 diabetes there are no approved oral agents, although some people use metformin off label. But I would predict if the numbers we saw with dapagliflozin in this short-term study persist out to 3 months, it would mean a reduction in hemoglobin A1c of 0.7-1.0 percentage points in type 1 diabetic patients who started at 8.0%," Dr. Robert R. Henry said in an interview at the annual scientific sessions of the American Diabetes Association.
He presented a small, proof-of-concept, multicenter, double-blind, phase IIa study aimed at establishing the safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT-2) inhibitor, in the management of patients with type 1 diabetes on background insulin. The study also showed early evidence of efficacy.
The five-center trial involved 62 patients who had suboptimally controlled type 1 diabetes despite being on basal bolus insulin or continuous infusion pump therapy. Their mean baseline HbA1c was 8.5%. Since this was the first study of an SGLT-2 inhibitor in patients with type 1 diabetes, it was conducted on an inpatient basis. For the first 3 days, patients were stabilized. Then they were randomized to insulin plus dapagliflozin at 1, 2.5, 5, or 10 mg once daily or to placebo.
The SGLT-2 inhibitor–treated patients demonstrated a dose-dependent increase in 24-hour urine glucose excretion. At the most effective 5- and 10-mg doses, by day 7 the 24-hour urine glucose excretion reached 84 and 100 g, respectively, representing mean 72-g and 89-g increases from baseline, compared to a 22-g decrease from baseline in the placebo group. Yet the dapagliflozin-treated patients showed no significant change in daily urine volume.
Continuous glucose monitoring showed mean 30- and 41-mg/dL reductions in daily average blood glucose levels in the 5- and 10-mg dapagliflozin groups, from a baseline of 174 mg/dL. Daily blood glucose variability decreased by 16%-25% as well, reported Dr. Henry, professor of medicine at the University of California, San Diego, and chief of the section of endocrinology, metabolism, and diabetes at Veterans Affairs Health Care System in San Diego.
Total daily insulin dosing on day 7 was down from baseline by 19% and 16%, respectively, in the 5- and 10-mg dapagliflozin-treated patients.
"This is just what I would have predicted, that dapagliflozin would be very effective in type 1 diabetes," he said.
Hypoglycemia was common in all study arms. The one case of serious hypoglycemia occurred in the dapagliflozin 10-mg group and led to study discontinuation. Two genital infections occurred in the dapagliflozin-treated patients.
Larger, longer-term clinical trials of the SGLT-2 inhibitor in patients with type 1 diabetes are planned.
The FDA initially rejected the New Drug Application that would have made dapagliflozin the first SGLT-2 inhibitor approved for the treatment of patients with type 2 diabetes. The agency requested more data on the risk/benefit ratio. Since then, dapagliflozin received marketing approval in the European Union, and the FDA granted approval to canagliflozin (Invokana) as the first SGLT-2 inhibitor to reach the U.S. market. AstraZeneca and Bristol-Myers Squibb have resubmitted the application for dapagliflozin with additional data. The FDA has indicated a decision will be announced by January.
Dr. Henry reported serving as an adviser to AstraZeneca and Bristol-Myers Squibb, which sponsored the study.
CHICAGO – Even as dapagliflozin’s resubmitted application for marketing approval for treatment of type 2 diabetes is being scrutinized by the Food and Drug Administration, the drug is under study as a novel potential oral therapy for type 1 diabetes.
"For type 1 diabetes there are no approved oral agents, although some people use metformin off label. But I would predict if the numbers we saw with dapagliflozin in this short-term study persist out to 3 months, it would mean a reduction in hemoglobin A1c of 0.7-1.0 percentage points in type 1 diabetic patients who started at 8.0%," Dr. Robert R. Henry said in an interview at the annual scientific sessions of the American Diabetes Association.
He presented a small, proof-of-concept, multicenter, double-blind, phase IIa study aimed at establishing the safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT-2) inhibitor, in the management of patients with type 1 diabetes on background insulin. The study also showed early evidence of efficacy.
The five-center trial involved 62 patients who had suboptimally controlled type 1 diabetes despite being on basal bolus insulin or continuous infusion pump therapy. Their mean baseline HbA1c was 8.5%. Since this was the first study of an SGLT-2 inhibitor in patients with type 1 diabetes, it was conducted on an inpatient basis. For the first 3 days, patients were stabilized. Then they were randomized to insulin plus dapagliflozin at 1, 2.5, 5, or 10 mg once daily or to placebo.
The SGLT-2 inhibitor–treated patients demonstrated a dose-dependent increase in 24-hour urine glucose excretion. At the most effective 5- and 10-mg doses, by day 7 the 24-hour urine glucose excretion reached 84 and 100 g, respectively, representing mean 72-g and 89-g increases from baseline, compared to a 22-g decrease from baseline in the placebo group. Yet the dapagliflozin-treated patients showed no significant change in daily urine volume.
Continuous glucose monitoring showed mean 30- and 41-mg/dL reductions in daily average blood glucose levels in the 5- and 10-mg dapagliflozin groups, from a baseline of 174 mg/dL. Daily blood glucose variability decreased by 16%-25% as well, reported Dr. Henry, professor of medicine at the University of California, San Diego, and chief of the section of endocrinology, metabolism, and diabetes at Veterans Affairs Health Care System in San Diego.
Total daily insulin dosing on day 7 was down from baseline by 19% and 16%, respectively, in the 5- and 10-mg dapagliflozin-treated patients.
"This is just what I would have predicted, that dapagliflozin would be very effective in type 1 diabetes," he said.
Hypoglycemia was common in all study arms. The one case of serious hypoglycemia occurred in the dapagliflozin 10-mg group and led to study discontinuation. Two genital infections occurred in the dapagliflozin-treated patients.
Larger, longer-term clinical trials of the SGLT-2 inhibitor in patients with type 1 diabetes are planned.
The FDA initially rejected the New Drug Application that would have made dapagliflozin the first SGLT-2 inhibitor approved for the treatment of patients with type 2 diabetes. The agency requested more data on the risk/benefit ratio. Since then, dapagliflozin received marketing approval in the European Union, and the FDA granted approval to canagliflozin (Invokana) as the first SGLT-2 inhibitor to reach the U.S. market. AstraZeneca and Bristol-Myers Squibb have resubmitted the application for dapagliflozin with additional data. The FDA has indicated a decision will be announced by January.
Dr. Henry reported serving as an adviser to AstraZeneca and Bristol-Myers Squibb, which sponsored the study.
CHICAGO – Even as dapagliflozin’s resubmitted application for marketing approval for treatment of type 2 diabetes is being scrutinized by the Food and Drug Administration, the drug is under study as a novel potential oral therapy for type 1 diabetes.
"For type 1 diabetes there are no approved oral agents, although some people use metformin off label. But I would predict if the numbers we saw with dapagliflozin in this short-term study persist out to 3 months, it would mean a reduction in hemoglobin A1c of 0.7-1.0 percentage points in type 1 diabetic patients who started at 8.0%," Dr. Robert R. Henry said in an interview at the annual scientific sessions of the American Diabetes Association.
He presented a small, proof-of-concept, multicenter, double-blind, phase IIa study aimed at establishing the safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT-2) inhibitor, in the management of patients with type 1 diabetes on background insulin. The study also showed early evidence of efficacy.
The five-center trial involved 62 patients who had suboptimally controlled type 1 diabetes despite being on basal bolus insulin or continuous infusion pump therapy. Their mean baseline HbA1c was 8.5%. Since this was the first study of an SGLT-2 inhibitor in patients with type 1 diabetes, it was conducted on an inpatient basis. For the first 3 days, patients were stabilized. Then they were randomized to insulin plus dapagliflozin at 1, 2.5, 5, or 10 mg once daily or to placebo.
The SGLT-2 inhibitor–treated patients demonstrated a dose-dependent increase in 24-hour urine glucose excretion. At the most effective 5- and 10-mg doses, by day 7 the 24-hour urine glucose excretion reached 84 and 100 g, respectively, representing mean 72-g and 89-g increases from baseline, compared to a 22-g decrease from baseline in the placebo group. Yet the dapagliflozin-treated patients showed no significant change in daily urine volume.
Continuous glucose monitoring showed mean 30- and 41-mg/dL reductions in daily average blood glucose levels in the 5- and 10-mg dapagliflozin groups, from a baseline of 174 mg/dL. Daily blood glucose variability decreased by 16%-25% as well, reported Dr. Henry, professor of medicine at the University of California, San Diego, and chief of the section of endocrinology, metabolism, and diabetes at Veterans Affairs Health Care System in San Diego.
Total daily insulin dosing on day 7 was down from baseline by 19% and 16%, respectively, in the 5- and 10-mg dapagliflozin-treated patients.
"This is just what I would have predicted, that dapagliflozin would be very effective in type 1 diabetes," he said.
Hypoglycemia was common in all study arms. The one case of serious hypoglycemia occurred in the dapagliflozin 10-mg group and led to study discontinuation. Two genital infections occurred in the dapagliflozin-treated patients.
Larger, longer-term clinical trials of the SGLT-2 inhibitor in patients with type 1 diabetes are planned.
The FDA initially rejected the New Drug Application that would have made dapagliflozin the first SGLT-2 inhibitor approved for the treatment of patients with type 2 diabetes. The agency requested more data on the risk/benefit ratio. Since then, dapagliflozin received marketing approval in the European Union, and the FDA granted approval to canagliflozin (Invokana) as the first SGLT-2 inhibitor to reach the U.S. market. AstraZeneca and Bristol-Myers Squibb have resubmitted the application for dapagliflozin with additional data. The FDA has indicated a decision will be announced by January.
Dr. Henry reported serving as an adviser to AstraZeneca and Bristol-Myers Squibb, which sponsored the study.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Patients with suboptimally controlled type 1 diabetes on insulin therapy who were randomized to add-on once-daily oral dapagliflozin excreted up to 100 g of glucose in their urine daily, while their average 24-hour blood glucose level fell by 41 mg/dL.
Data source: A randomized, double-blind, placebo-controlled, multicenter, short-term, inpatient, phase IIa study in 62 patients with type 1 diabetes suboptimally controlled with basal bolus insulin or insulin pump therapy.
Disclosures: AstraZeneca and Bristol-Myers Squibb sponsored the study. The presenter is an adviser to those pharmaceutical companies and about a dozen others.
Novel GLP-1 agonist dulaglutide wins AWARD for type 2 diabetes
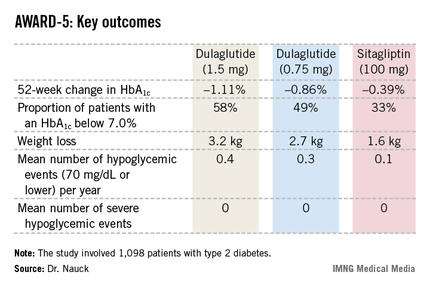
CHICAGO – Dulaglutide, an investigational once-weekly long-acting glucagonlike peptide–1 receptor agonist, provided superior glycemic control compared with sitagliptin in patients with type 2 diabetes in the phase III AWARD-5 trial.
In this 52-week, 1,098-patient randomized trial, both the 0.75- and 1.5-mg doses of once-weekly dulaglutide proved superior to oral sitagliptin (Januvia) at the standard dose of 100 mg/day in key efficacy endpoints. These outcomes included magnitude of hemoglobin A1c reduction, achievement of guideline-recommended HbA1c targets, and weight loss, Dr. Michael A. Nauck reported at the annual scientific sessions of the American Diabetes Association.
Dulaglutide is a recombinant glucagonlike peptide–1 (GLP-1) fusion protein linking a human GLP-1 peptide analogue to a large protein that gives the medication a lengthy half-life of 5 days. Metabolism of dulaglutide is not renally dependent, making the drug a logical candidate for future study in type 2 diabetic patients with renal impairment, observed Dr. Nauck, head of the Bad Lauterberg (Germany) Diabetes Center.
AWARD-5: Dulaglutide vs. sitagliptin
The fifth Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD-5) was one of three phase III clinical trials in the AWARD program presented at the meeting. In AWARD-1, the once-weekly injectable GLP-1 agonist demonstrated superior glycemic control compared with twice-daily exenatide (Byetta) in type 2 diabetic patients not adequately controlled with full-dose metformin and pioglitazone. AWARD-3 showed dulaglutide to be superior to metformin in patients with early type 2 diabetes.
The 1,098 participants in AWARD-5 had a mean baseline HbA1c of 8.1% despite being on metformin and, in one-quarter of cases, an additional oral antidiabetic agent. They stayed on their previous oral medication during the trial. Their mean baseline body mass index was 31 kg/m2, and they averaged a 7-year history of diabetes.
The primary objective in AWARD-5 was to establish that dulaglutide was noninferior to sitagliptin in its ability to reduce HbA1c from baseline through 52 weeks. But in fact, Dr. Nauck reported, both doses of dulaglutide exceeded that standard and actually achieved statistically significant and clinically meaningful superiority to the dipeptidyl peptidase-4 inhibitor (see chart).
With regard to safety issues, the endocrinologist noted that hypoglycemia rates didn’t vary significantly between the study arms. No cases of pancreatitis or pancreatic cancer occurred in dulaglutide-treated patients. There were three cases of pancreatitis in the sitagliptin group.
Gastrointestinal side effects were common among recipients of the once-weekly GLP-1 agonist. The incidence of nausea was 17% in patients who received dulaglutide at 1.5 mg, 14% with 0.75 mg, and 5% with sitagliptin. Diarrhea occurred in 15% and 10% of patients on the high- and low-dose dulaglutide regimens, respectively, compared with 3% on sitagliptin. The incidence of vomiting was 13% with dulaglutide at 1.5 mg, 8% at 0.75 mg, and 2% with sitagliptin. However, the frequency of dulaglutide-related GI side effects dropped off markedly after the first 4-8 weeks, the side effects were mostly mild to moderate in nature, and very few patients dropped out of the study due to GI symptoms, according to Dr. Nauck.
AWARD-1: Dulaglutide vs. exenatide
In a separate presentation, Dr. Carol Wysham reported on 978 patients with type 2 diabetes in the 52-week, phase III AWARD-1 trial who were randomized to dulaglutide at 1.5 or 0.75 mg, the injectable GLP-1 agonist exenatide at 10 mcg twice daily, or placebo. Their baseline HbA1c was 8.07% despite being on metformin and pioglitazone.
The primary endpoint was change in HbA1c at 26 weeks. The reduction was significantly bigger in the two dulaglutide study arms than with exenatide: –1.51% compared with baseline in the dulaglutide 1.5-mg arm, –1.3% with dulaglutide 0.75 mg, –0.99% with exenatide, and –0.46% with placebo. The proportion of patients with an HbA1c of 6.5% or less at 26 weeks was 64% with dulaglutide 1.5 mg, 54% with the long-acting agent at 0.75 mg, 39% with sitagliptin, and 26% in placebo-treated controls.
These results held up at the 52-week mark as well. At that time, 71% of the dulaglutide 1.5-mg group had an HbA1c below 7.0%, as did 59% of patients on dulaglutide 0.75 mg and 49% on exenatide.
As in the other AWARD trials, most of the reduction in body weight and fasting plasma glucose in dulaglutide-treated patients occurred in the first 2 weeks of treatment. The early benefits were then maintained through 52 weeks, observed Dr. Wysham, an endocrinologist in Spokane, Wash.
AWARD-3: Dulaglutide vs. metformin
Dr. Guillermo E. Umpierrez presented the results of AWARD-3, which involved 807 patients with early type 2 diabetes as evidenced by their mean 2.6-year disease history. Their baseline HbA1c was 7.6% with diet and exercise alone or in combination with a single, low-dose, oral antidiabetic medication. Participants were randomized to dulaglutide at 1.5 or 0.75 mg or to metformin at 1,000 mg twice daily.
The primary endpoint was change in HbA1c over 26 weeks. Both doses of dulaglutide proved superior to metformin, with reductions of 0.78% with dulaglutide at 1.5 mg and 0.71% at 0.75 mg, compared with 0.56% with metformin, reported Dr. Umpierrez, professor of medicine at Emory University and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
Session chair Dr. Julio Rosenstock didn’t buy Dr. Umpierrez’ conclusion that AWARD-3 proved dulaglutide to be superior to metformin. He argued that even if the differences in HbA1c reduction between the dulaglutide- and metformin-treated patients were statistically significant, they were too small to be clinically meaningful. "I don’t think this is clinical superiority. You’ve presented evidence that dulaglutide is comparable in efficacy to metformin in early type 2 diabetes. I see it as an option for patients who can’t tolerate metformin," commented Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center.
The AWARD trials were supported by Eli Lilly, which anticipates applying to the Food and Drug Administration for marketing approval of dulaglutide before the end of the year. All three presenters reported receiving research grants from Eli Lilly and other pharmaceutical companies. Drs. Wysham and Nauck are also on the Eli Lilly advisory board.
CHICAGO – Dulaglutide, an investigational once-weekly long-acting glucagonlike peptide–1 receptor agonist, provided superior glycemic control compared with sitagliptin in patients with type 2 diabetes in the phase III AWARD-5 trial.
In this 52-week, 1,098-patient randomized trial, both the 0.75- and 1.5-mg doses of once-weekly dulaglutide proved superior to oral sitagliptin (Januvia) at the standard dose of 100 mg/day in key efficacy endpoints. These outcomes included magnitude of hemoglobin A1c reduction, achievement of guideline-recommended HbA1c targets, and weight loss, Dr. Michael A. Nauck reported at the annual scientific sessions of the American Diabetes Association.

Dulaglutide is a recombinant glucagonlike peptide–1 (GLP-1) fusion protein linking a human GLP-1 peptide analogue to a large protein that gives the medication a lengthy half-life of 5 days. Metabolism of dulaglutide is not renally dependent, making the drug a logical candidate for future study in type 2 diabetic patients with renal impairment, observed Dr. Nauck, head of the Bad Lauterberg (Germany) Diabetes Center.
AWARD-5: Dulaglutide vs. sitagliptin
The fifth Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD-5) was one of three phase III clinical trials in the AWARD program presented at the meeting. In AWARD-1, the once-weekly injectable GLP-1 agonist demonstrated superior glycemic control compared with twice-daily exenatide (Byetta) in type 2 diabetic patients not adequately controlled with full-dose metformin and pioglitazone. AWARD-3 showed dulaglutide to be superior to metformin in patients with early type 2 diabetes.
The 1,098 participants in AWARD-5 had a mean baseline HbA1c of 8.1% despite being on metformin and, in one-quarter of cases, an additional oral antidiabetic agent. They stayed on their previous oral medication during the trial. Their mean baseline body mass index was 31 kg/m2, and they averaged a 7-year history of diabetes.
The primary objective in AWARD-5 was to establish that dulaglutide was noninferior to sitagliptin in its ability to reduce HbA1c from baseline through 52 weeks. But in fact, Dr. Nauck reported, both doses of dulaglutide exceeded that standard and actually achieved statistically significant and clinically meaningful superiority to the dipeptidyl peptidase-4 inhibitor (see chart).
With regard to safety issues, the endocrinologist noted that hypoglycemia rates didn’t vary significantly between the study arms. No cases of pancreatitis or pancreatic cancer occurred in dulaglutide-treated patients. There were three cases of pancreatitis in the sitagliptin group.
Gastrointestinal side effects were common among recipients of the once-weekly GLP-1 agonist. The incidence of nausea was 17% in patients who received dulaglutide at 1.5 mg, 14% with 0.75 mg, and 5% with sitagliptin. Diarrhea occurred in 15% and 10% of patients on the high- and low-dose dulaglutide regimens, respectively, compared with 3% on sitagliptin. The incidence of vomiting was 13% with dulaglutide at 1.5 mg, 8% at 0.75 mg, and 2% with sitagliptin. However, the frequency of dulaglutide-related GI side effects dropped off markedly after the first 4-8 weeks, the side effects were mostly mild to moderate in nature, and very few patients dropped out of the study due to GI symptoms, according to Dr. Nauck.
AWARD-1: Dulaglutide vs. exenatide
In a separate presentation, Dr. Carol Wysham reported on 978 patients with type 2 diabetes in the 52-week, phase III AWARD-1 trial who were randomized to dulaglutide at 1.5 or 0.75 mg, the injectable GLP-1 agonist exenatide at 10 mcg twice daily, or placebo. Their baseline HbA1c was 8.07% despite being on metformin and pioglitazone.
The primary endpoint was change in HbA1c at 26 weeks. The reduction was significantly bigger in the two dulaglutide study arms than with exenatide: –1.51% compared with baseline in the dulaglutide 1.5-mg arm, –1.3% with dulaglutide 0.75 mg, –0.99% with exenatide, and –0.46% with placebo. The proportion of patients with an HbA1c of 6.5% or less at 26 weeks was 64% with dulaglutide 1.5 mg, 54% with the long-acting agent at 0.75 mg, 39% with sitagliptin, and 26% in placebo-treated controls.
These results held up at the 52-week mark as well. At that time, 71% of the dulaglutide 1.5-mg group had an HbA1c below 7.0%, as did 59% of patients on dulaglutide 0.75 mg and 49% on exenatide.
As in the other AWARD trials, most of the reduction in body weight and fasting plasma glucose in dulaglutide-treated patients occurred in the first 2 weeks of treatment. The early benefits were then maintained through 52 weeks, observed Dr. Wysham, an endocrinologist in Spokane, Wash.
AWARD-3: Dulaglutide vs. metformin
Dr. Guillermo E. Umpierrez presented the results of AWARD-3, which involved 807 patients with early type 2 diabetes as evidenced by their mean 2.6-year disease history. Their baseline HbA1c was 7.6% with diet and exercise alone or in combination with a single, low-dose, oral antidiabetic medication. Participants were randomized to dulaglutide at 1.5 or 0.75 mg or to metformin at 1,000 mg twice daily.
The primary endpoint was change in HbA1c over 26 weeks. Both doses of dulaglutide proved superior to metformin, with reductions of 0.78% with dulaglutide at 1.5 mg and 0.71% at 0.75 mg, compared with 0.56% with metformin, reported Dr. Umpierrez, professor of medicine at Emory University and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
Session chair Dr. Julio Rosenstock didn’t buy Dr. Umpierrez’ conclusion that AWARD-3 proved dulaglutide to be superior to metformin. He argued that even if the differences in HbA1c reduction between the dulaglutide- and metformin-treated patients were statistically significant, they were too small to be clinically meaningful. "I don’t think this is clinical superiority. You’ve presented evidence that dulaglutide is comparable in efficacy to metformin in early type 2 diabetes. I see it as an option for patients who can’t tolerate metformin," commented Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center.
The AWARD trials were supported by Eli Lilly, which anticipates applying to the Food and Drug Administration for marketing approval of dulaglutide before the end of the year. All three presenters reported receiving research grants from Eli Lilly and other pharmaceutical companies. Drs. Wysham and Nauck are also on the Eli Lilly advisory board.
CHICAGO – Dulaglutide, an investigational once-weekly long-acting glucagonlike peptide–1 receptor agonist, provided superior glycemic control compared with sitagliptin in patients with type 2 diabetes in the phase III AWARD-5 trial.
In this 52-week, 1,098-patient randomized trial, both the 0.75- and 1.5-mg doses of once-weekly dulaglutide proved superior to oral sitagliptin (Januvia) at the standard dose of 100 mg/day in key efficacy endpoints. These outcomes included magnitude of hemoglobin A1c reduction, achievement of guideline-recommended HbA1c targets, and weight loss, Dr. Michael A. Nauck reported at the annual scientific sessions of the American Diabetes Association.

Dulaglutide is a recombinant glucagonlike peptide–1 (GLP-1) fusion protein linking a human GLP-1 peptide analogue to a large protein that gives the medication a lengthy half-life of 5 days. Metabolism of dulaglutide is not renally dependent, making the drug a logical candidate for future study in type 2 diabetic patients with renal impairment, observed Dr. Nauck, head of the Bad Lauterberg (Germany) Diabetes Center.
AWARD-5: Dulaglutide vs. sitagliptin
The fifth Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD-5) was one of three phase III clinical trials in the AWARD program presented at the meeting. In AWARD-1, the once-weekly injectable GLP-1 agonist demonstrated superior glycemic control compared with twice-daily exenatide (Byetta) in type 2 diabetic patients not adequately controlled with full-dose metformin and pioglitazone. AWARD-3 showed dulaglutide to be superior to metformin in patients with early type 2 diabetes.
The 1,098 participants in AWARD-5 had a mean baseline HbA1c of 8.1% despite being on metformin and, in one-quarter of cases, an additional oral antidiabetic agent. They stayed on their previous oral medication during the trial. Their mean baseline body mass index was 31 kg/m2, and they averaged a 7-year history of diabetes.
The primary objective in AWARD-5 was to establish that dulaglutide was noninferior to sitagliptin in its ability to reduce HbA1c from baseline through 52 weeks. But in fact, Dr. Nauck reported, both doses of dulaglutide exceeded that standard and actually achieved statistically significant and clinically meaningful superiority to the dipeptidyl peptidase-4 inhibitor (see chart).
With regard to safety issues, the endocrinologist noted that hypoglycemia rates didn’t vary significantly between the study arms. No cases of pancreatitis or pancreatic cancer occurred in dulaglutide-treated patients. There were three cases of pancreatitis in the sitagliptin group.
Gastrointestinal side effects were common among recipients of the once-weekly GLP-1 agonist. The incidence of nausea was 17% in patients who received dulaglutide at 1.5 mg, 14% with 0.75 mg, and 5% with sitagliptin. Diarrhea occurred in 15% and 10% of patients on the high- and low-dose dulaglutide regimens, respectively, compared with 3% on sitagliptin. The incidence of vomiting was 13% with dulaglutide at 1.5 mg, 8% at 0.75 mg, and 2% with sitagliptin. However, the frequency of dulaglutide-related GI side effects dropped off markedly after the first 4-8 weeks, the side effects were mostly mild to moderate in nature, and very few patients dropped out of the study due to GI symptoms, according to Dr. Nauck.
AWARD-1: Dulaglutide vs. exenatide
In a separate presentation, Dr. Carol Wysham reported on 978 patients with type 2 diabetes in the 52-week, phase III AWARD-1 trial who were randomized to dulaglutide at 1.5 or 0.75 mg, the injectable GLP-1 agonist exenatide at 10 mcg twice daily, or placebo. Their baseline HbA1c was 8.07% despite being on metformin and pioglitazone.
The primary endpoint was change in HbA1c at 26 weeks. The reduction was significantly bigger in the two dulaglutide study arms than with exenatide: –1.51% compared with baseline in the dulaglutide 1.5-mg arm, –1.3% with dulaglutide 0.75 mg, –0.99% with exenatide, and –0.46% with placebo. The proportion of patients with an HbA1c of 6.5% or less at 26 weeks was 64% with dulaglutide 1.5 mg, 54% with the long-acting agent at 0.75 mg, 39% with sitagliptin, and 26% in placebo-treated controls.
These results held up at the 52-week mark as well. At that time, 71% of the dulaglutide 1.5-mg group had an HbA1c below 7.0%, as did 59% of patients on dulaglutide 0.75 mg and 49% on exenatide.
As in the other AWARD trials, most of the reduction in body weight and fasting plasma glucose in dulaglutide-treated patients occurred in the first 2 weeks of treatment. The early benefits were then maintained through 52 weeks, observed Dr. Wysham, an endocrinologist in Spokane, Wash.
AWARD-3: Dulaglutide vs. metformin
Dr. Guillermo E. Umpierrez presented the results of AWARD-3, which involved 807 patients with early type 2 diabetes as evidenced by their mean 2.6-year disease history. Their baseline HbA1c was 7.6% with diet and exercise alone or in combination with a single, low-dose, oral antidiabetic medication. Participants were randomized to dulaglutide at 1.5 or 0.75 mg or to metformin at 1,000 mg twice daily.
The primary endpoint was change in HbA1c over 26 weeks. Both doses of dulaglutide proved superior to metformin, with reductions of 0.78% with dulaglutide at 1.5 mg and 0.71% at 0.75 mg, compared with 0.56% with metformin, reported Dr. Umpierrez, professor of medicine at Emory University and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
Session chair Dr. Julio Rosenstock didn’t buy Dr. Umpierrez’ conclusion that AWARD-3 proved dulaglutide to be superior to metformin. He argued that even if the differences in HbA1c reduction between the dulaglutide- and metformin-treated patients were statistically significant, they were too small to be clinically meaningful. "I don’t think this is clinical superiority. You’ve presented evidence that dulaglutide is comparable in efficacy to metformin in early type 2 diabetes. I see it as an option for patients who can’t tolerate metformin," commented Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center.
The AWARD trials were supported by Eli Lilly, which anticipates applying to the Food and Drug Administration for marketing approval of dulaglutide before the end of the year. All three presenters reported receiving research grants from Eli Lilly and other pharmaceutical companies. Drs. Wysham and Nauck are also on the Eli Lilly advisory board.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
4B trial showcases novel strategy in uncontrolled type 2 diabetes
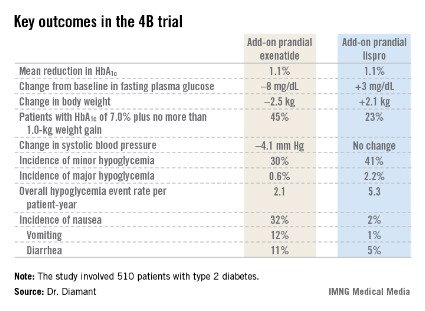
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.
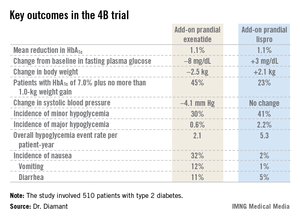
The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.

The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
CHICAGO – Adding a premeal short-acting glucagonlike peptide–1 agonist is an attractive alternative to add-on prandial insulin when intensification of basal insulin no longer controls type 2 diabetes, according to the findings of the 4B trial.
"Addition of prandial insulin has been the gold standard. But we know that that is often associated with a high risk of hypoglycemia, weight gain, and low patient acceptance, so we need alternative treatment approaches," principal investigator Dr. Michaela Diamant said, in explaining the study rationale at the annual scientific sessions of the American Diabetes Association.

The 4B (basal insulin glargine and exenatide b.i.d. treatment or basal insulin glargine and mealtime bolus insulin lispro treatment) trial was the first study to evaluate a prandial glucagonlike peptide–1 agonist as such an approach. The open-label, prospective, randomized phase IIIb study involved 510 type 2 diabetic patients in 17 countries. All had a hemoglobin A1c level above 7.0% despite metformin plus 12 weeks of basal insulin glargine (Lantus) intensification using the standardized titration algorithm known as INITIATE. Indeed, their mean HbA1c level at randomization was 8.3%.
Participants were randomized to 30 weeks of either add-on, twice-daily premeal exenatide (Byetta) or mealtime bolus insulin lispro (Humalog) three times daily. The mean daily insulin lispro dose was 42.1 U. Eighty percent of patients in the exenatide group ended up on 10 mcg twice daily, with the rest on half that dose, explained Dr. Diamant of the Free University of Amsterdam.
The key study findings: The two treatment strategies resulted in similar HbA1c reductions, but the exenatide group had a lower final mean fasting plasma glucose level, a significant advantage in terms of change in body weight, less daytime hypoglycemia, lower systolic blood pressure, better patient treatment-satisfaction scores, and much more nausea.
The 4B trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: Patients with inadequately controlled type 2 diabetes, despite their being on metformin and optimized basal insulin, had better outcomes in multiple domains if they received add-on premeal exenatide twice daily than with add-on prandial insulin lispro three times daily.
Data Source: A phase IIIb, open-label, prospective randomized trial involving 510 type 2 diabetic patients with inadequate glycemic control.
Disclosures: The trial was supported by Eli Lilly. Dr. Diamant reported receiving research grants from as well as serving as a consultant to and an advisory board member for Eli Lilly and other pharmaceutical companies.
Education improved impaired hypoglycemia awareness
CHICAGO – Patients with type 1 diabetes who had impaired awareness of hypoglycemia and were provided with education and support, had equal biochemical outcomes whether they were on insulin pump therapy or daily injections.
Researchers also found that the outcomes were equal for patients whether they were on conventional or real-time glucose monitoring.
Impaired awareness of hypoglycemia, or IAH, affects roughly 20% of adults with type 1 diabetes. Also, severe hypoglycemia is six times more likely in these adults, said Dr. Stuart A. Little of Newcastle upon Tyne, England, who presented the results of Hypo COMPaSS, a 24-week, randomized controlled trial of 96 participants, at the annual scientific sessions of the American Diabetes Association.
"Sometimes patients with impaired awareness say that they don’t even feel it, and they think it’s okay," said Marjorie Cypress, Ph.D., the 2013 president-elect of ADA’s health care and education committee. "They need to learn that it’s not okay, that they’re not getting those warning signs, but they’re losing glucose from their brain."
Most of the participants in the trial were women (63%), their mean age was 49 years, their diabetes duration was about 29 years, and their mean HbA1c level was 66 mmol/mol, or about 8.2%.
Dr. Little and his colleagues randomized the 96 participants to a multiple daily injection (MDI) group (50 patients) and an insulin pump therapy (CSII) group (46). In the MDI group, 24 patients did not have real-time (RT) continuous glucose monitoring, and 26 did. And in the CSII group, 24 patients had no RT, and 22 did.
The primary endpoint of the study was the difference in 24-week Gold score, with an 80% power to detect a Gold score difference of 1.1 on a 7-point Likert scale.
Secondary endpoints included overall glycemic control and patient-reported outcomes.
All patients received equal support and had a treatment goal of rigorous avoidance of biochemical hypoglycemia without relaxation of overall hemoglobin A1c. They all had Gold scores of at least 4, indicating IAH.
Within 4 weeks, the patients’ biochemical hypoglycemia was reduced significantly, from 3.7% to 1.7% of the time, from 53 minutes/day to under half an hour. The reduction was maintained throughout the study.
The median Gold score also showed a statistically significant reduction by week 24 among all participants, dropping from 5 at baseline to 4 at the end of the study period.
There was also a dramatic reduction in severe hypoglycemic events, said Dr. Little. While 92% of the patients were affected by the condition the year before the study, and 77% were affected 6 months before the trial, only 19% were affected during the trial.
Fear of hypoglycemia was significantly reduced and treatment satisfaction improved among all participants. The mean HbA1c did not change throughout the study among the participants.
When the investigators compared the MDI and CSII groups, the Gold score and severe hypoglycemia were not significantly different, nor were the HbA1c levels or insulin units. And although the fear of hypoglycemia did not differ significantly between the two groups, the treatment satisfaction was significantly higher in the CSII group.
Comparing the patients based on real-time glucose monitoring or self-monitoring showed no statistically significant difference in Gold score, severe hypoglycemia, HbA1c levels, or insulin units. There were also no significant differences in fear of hypoglycemia and treatment satisfaction.
"The bottom line is, if you give people education about hypoglycemia, they do better. We’ve seen this before in other studies. Teach people, and they can avoid hypoglycemia," said Dr. Cypress.
Dr. Little and Dr. Cypress had no disclosures.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes who had impaired awareness of hypoglycemia and were provided with education and support, had equal biochemical outcomes whether they were on insulin pump therapy or daily injections.
Researchers also found that the outcomes were equal for patients whether they were on conventional or real-time glucose monitoring.
Impaired awareness of hypoglycemia, or IAH, affects roughly 20% of adults with type 1 diabetes. Also, severe hypoglycemia is six times more likely in these adults, said Dr. Stuart A. Little of Newcastle upon Tyne, England, who presented the results of Hypo COMPaSS, a 24-week, randomized controlled trial of 96 participants, at the annual scientific sessions of the American Diabetes Association.
"Sometimes patients with impaired awareness say that they don’t even feel it, and they think it’s okay," said Marjorie Cypress, Ph.D., the 2013 president-elect of ADA’s health care and education committee. "They need to learn that it’s not okay, that they’re not getting those warning signs, but they’re losing glucose from their brain."
Most of the participants in the trial were women (63%), their mean age was 49 years, their diabetes duration was about 29 years, and their mean HbA1c level was 66 mmol/mol, or about 8.2%.
Dr. Little and his colleagues randomized the 96 participants to a multiple daily injection (MDI) group (50 patients) and an insulin pump therapy (CSII) group (46). In the MDI group, 24 patients did not have real-time (RT) continuous glucose monitoring, and 26 did. And in the CSII group, 24 patients had no RT, and 22 did.
The primary endpoint of the study was the difference in 24-week Gold score, with an 80% power to detect a Gold score difference of 1.1 on a 7-point Likert scale.
Secondary endpoints included overall glycemic control and patient-reported outcomes.
All patients received equal support and had a treatment goal of rigorous avoidance of biochemical hypoglycemia without relaxation of overall hemoglobin A1c. They all had Gold scores of at least 4, indicating IAH.
Within 4 weeks, the patients’ biochemical hypoglycemia was reduced significantly, from 3.7% to 1.7% of the time, from 53 minutes/day to under half an hour. The reduction was maintained throughout the study.
The median Gold score also showed a statistically significant reduction by week 24 among all participants, dropping from 5 at baseline to 4 at the end of the study period.
There was also a dramatic reduction in severe hypoglycemic events, said Dr. Little. While 92% of the patients were affected by the condition the year before the study, and 77% were affected 6 months before the trial, only 19% were affected during the trial.
Fear of hypoglycemia was significantly reduced and treatment satisfaction improved among all participants. The mean HbA1c did not change throughout the study among the participants.
When the investigators compared the MDI and CSII groups, the Gold score and severe hypoglycemia were not significantly different, nor were the HbA1c levels or insulin units. And although the fear of hypoglycemia did not differ significantly between the two groups, the treatment satisfaction was significantly higher in the CSII group.
Comparing the patients based on real-time glucose monitoring or self-monitoring showed no statistically significant difference in Gold score, severe hypoglycemia, HbA1c levels, or insulin units. There were also no significant differences in fear of hypoglycemia and treatment satisfaction.
"The bottom line is, if you give people education about hypoglycemia, they do better. We’ve seen this before in other studies. Teach people, and they can avoid hypoglycemia," said Dr. Cypress.
Dr. Little and Dr. Cypress had no disclosures.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes who had impaired awareness of hypoglycemia and were provided with education and support, had equal biochemical outcomes whether they were on insulin pump therapy or daily injections.
Researchers also found that the outcomes were equal for patients whether they were on conventional or real-time glucose monitoring.
Impaired awareness of hypoglycemia, or IAH, affects roughly 20% of adults with type 1 diabetes. Also, severe hypoglycemia is six times more likely in these adults, said Dr. Stuart A. Little of Newcastle upon Tyne, England, who presented the results of Hypo COMPaSS, a 24-week, randomized controlled trial of 96 participants, at the annual scientific sessions of the American Diabetes Association.
"Sometimes patients with impaired awareness say that they don’t even feel it, and they think it’s okay," said Marjorie Cypress, Ph.D., the 2013 president-elect of ADA’s health care and education committee. "They need to learn that it’s not okay, that they’re not getting those warning signs, but they’re losing glucose from their brain."
Most of the participants in the trial were women (63%), their mean age was 49 years, their diabetes duration was about 29 years, and their mean HbA1c level was 66 mmol/mol, or about 8.2%.
Dr. Little and his colleagues randomized the 96 participants to a multiple daily injection (MDI) group (50 patients) and an insulin pump therapy (CSII) group (46). In the MDI group, 24 patients did not have real-time (RT) continuous glucose monitoring, and 26 did. And in the CSII group, 24 patients had no RT, and 22 did.
The primary endpoint of the study was the difference in 24-week Gold score, with an 80% power to detect a Gold score difference of 1.1 on a 7-point Likert scale.
Secondary endpoints included overall glycemic control and patient-reported outcomes.
All patients received equal support and had a treatment goal of rigorous avoidance of biochemical hypoglycemia without relaxation of overall hemoglobin A1c. They all had Gold scores of at least 4, indicating IAH.
Within 4 weeks, the patients’ biochemical hypoglycemia was reduced significantly, from 3.7% to 1.7% of the time, from 53 minutes/day to under half an hour. The reduction was maintained throughout the study.
The median Gold score also showed a statistically significant reduction by week 24 among all participants, dropping from 5 at baseline to 4 at the end of the study period.
There was also a dramatic reduction in severe hypoglycemic events, said Dr. Little. While 92% of the patients were affected by the condition the year before the study, and 77% were affected 6 months before the trial, only 19% were affected during the trial.
Fear of hypoglycemia was significantly reduced and treatment satisfaction improved among all participants. The mean HbA1c did not change throughout the study among the participants.
When the investigators compared the MDI and CSII groups, the Gold score and severe hypoglycemia were not significantly different, nor were the HbA1c levels or insulin units. And although the fear of hypoglycemia did not differ significantly between the two groups, the treatment satisfaction was significantly higher in the CSII group.
Comparing the patients based on real-time glucose monitoring or self-monitoring showed no statistically significant difference in Gold score, severe hypoglycemia, HbA1c levels, or insulin units. There were also no significant differences in fear of hypoglycemia and treatment satisfaction.
"The bottom line is, if you give people education about hypoglycemia, they do better. We’ve seen this before in other studies. Teach people, and they can avoid hypoglycemia," said Dr. Cypress.
Dr. Little and Dr. Cypress had no disclosures.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: The median Gold score measuring impaired awareness of hypoglycemia (IAH) dropped significantly among all participants, from 5 at baseline to 4 at week 24.
Data source: Hypo COMPaSS, a 24-week, randomized controlled trial of 96 participants with type 1 diabetes and IAH.
Disclosures: Dr. Little and Dr. Cypress had no disclosures.
Brain volume loss may be greater in type 1 diabetics with microangiopathy
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
AT ADA 2013
Major finding: The study group showed a larger percentage of whole-brain volume loss (–1.34%* vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
Data source: A total of 25 patients with type 1 diabetes who had microangiopathy, compared with 25 closely matched healthy participants.
Disclosures: Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
Discontinuation rates high with type 2 diabetes drugs
CHICAGO – Only 31% of patients with type 2 diabetes who were placed on a glucagonlike peptide-1 agonist persisted on the medication for at least 6 months in a very large national retrospective cohort study.
That’s significantly lower than the 39% rate of 6-month treatment persistence in patients placed on a dipeptidyl-peptidase-4 inhibitor, Carol E. Koro, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
The 12-month treatment discontinuation rate was 89% in patients placed on a GLP-1 agonist, compared with 82% for patients on a DPP-4 inhibitor and 84% among those placed on other antidiabetic agents, added Dr. Koro, an epidemiologist at GlaxoSmithKline in Research Triangle Park, N.C.
She presented a retrospective study examining treatment utilization patterns among 134,696 commercially insured type 2 diabetes patients placed on a GLP-1 agonist, 202,269 given a DPP-4 inhibitor, and 1,014,630 on other antidiabetic agents. The data source was the Truven Health Analytics MarketScan database for 2005-2011.
Nonadherence was defined by a prescription refill pattern indicating that less than 80% of a prescribed medication was being taken. The nonadherence rate was 9.6% in patients whose physicians prescribed a GLP-1 agonist, 5.4% in those placed on a DPP-4 inhibitor, and 4.4% in patients on other antidiabetic agents.
The median time to treatment discontinuation was 90 days in the GLP-1 agonist cohort, 120 days for the DPP-4 inhibitor cohort, and 90 days for patients assigned to other antidiabetic agents. The mean time to discontinuation was 178 days for the GLP-1 agonist group, 226 days for the DPP-4 inhibitor cohort, and 196 days for the group on other antidiabetic medications.
This was not a randomized trial, and the GLP-1 agonist cohort differed from the other two groups in important ways.
For example, they were more likely to be female: 57% of the GLP-1 agonist cohort were women, compared with 44% in the DPP-4 inhibitor cohort and 47% of patients on other antidiabetic agents. The GLP-1 agonist group also had a greater prevalence of obesity: 12%, compared with 8% in the DPP-4 inhibitor cohort and a similar figure among those on other antidiabetic agents. In addition, the GLP-1 agonist cohort had a higher baseline prevalence of microvascular complications.
Only 8% of patients placed on a GLP-1 agonist initiated the drug as new therapy; 78% started it as add-on therapy. In contrast, 17% of patients initiated a DPP-4 inhibitor as new therapy, while 59% employed the drug as add-on therapy.
The subgroup of the GLP-1 agonist cohort who initiated the drug as an add-on to basal insulin had a higher baseline prevalence of microvascular complications and greater use of antilipidemic drugs, antihypertensive agents, and antiplatelet agents than did those using the drug as an add-on to oral antidiabetic therapy. Nevertheless, the 6-month treatment persistence rates in the two subgroups were nearly identical.
The take-away message from this study is clear, Dr. Koro said: "These results demonstrate the need for improved persistence with GLP-1 agonist treatment."
She noted that other investigators have estimated that reducing the rate of nonadherence to antidiabetes medications could avoid 700,000 emergency department visits and 341,000 hospitalizations annually, with a resultant $4.7 billion annual savings in health care costs (Health Aff. [Millwood] 2012;31:1836-46).
GlaxoSmithKline funded Dr. Koro’s study. The company’s investigational GLP-1 agonist albiglutide is under review by the Food and Drug Administration.
CHICAGO – Only 31% of patients with type 2 diabetes who were placed on a glucagonlike peptide-1 agonist persisted on the medication for at least 6 months in a very large national retrospective cohort study.
That’s significantly lower than the 39% rate of 6-month treatment persistence in patients placed on a dipeptidyl-peptidase-4 inhibitor, Carol E. Koro, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
The 12-month treatment discontinuation rate was 89% in patients placed on a GLP-1 agonist, compared with 82% for patients on a DPP-4 inhibitor and 84% among those placed on other antidiabetic agents, added Dr. Koro, an epidemiologist at GlaxoSmithKline in Research Triangle Park, N.C.
She presented a retrospective study examining treatment utilization patterns among 134,696 commercially insured type 2 diabetes patients placed on a GLP-1 agonist, 202,269 given a DPP-4 inhibitor, and 1,014,630 on other antidiabetic agents. The data source was the Truven Health Analytics MarketScan database for 2005-2011.
Nonadherence was defined by a prescription refill pattern indicating that less than 80% of a prescribed medication was being taken. The nonadherence rate was 9.6% in patients whose physicians prescribed a GLP-1 agonist, 5.4% in those placed on a DPP-4 inhibitor, and 4.4% in patients on other antidiabetic agents.
The median time to treatment discontinuation was 90 days in the GLP-1 agonist cohort, 120 days for the DPP-4 inhibitor cohort, and 90 days for patients assigned to other antidiabetic agents. The mean time to discontinuation was 178 days for the GLP-1 agonist group, 226 days for the DPP-4 inhibitor cohort, and 196 days for the group on other antidiabetic medications.
This was not a randomized trial, and the GLP-1 agonist cohort differed from the other two groups in important ways.
For example, they were more likely to be female: 57% of the GLP-1 agonist cohort were women, compared with 44% in the DPP-4 inhibitor cohort and 47% of patients on other antidiabetic agents. The GLP-1 agonist group also had a greater prevalence of obesity: 12%, compared with 8% in the DPP-4 inhibitor cohort and a similar figure among those on other antidiabetic agents. In addition, the GLP-1 agonist cohort had a higher baseline prevalence of microvascular complications.
Only 8% of patients placed on a GLP-1 agonist initiated the drug as new therapy; 78% started it as add-on therapy. In contrast, 17% of patients initiated a DPP-4 inhibitor as new therapy, while 59% employed the drug as add-on therapy.
The subgroup of the GLP-1 agonist cohort who initiated the drug as an add-on to basal insulin had a higher baseline prevalence of microvascular complications and greater use of antilipidemic drugs, antihypertensive agents, and antiplatelet agents than did those using the drug as an add-on to oral antidiabetic therapy. Nevertheless, the 6-month treatment persistence rates in the two subgroups were nearly identical.
The take-away message from this study is clear, Dr. Koro said: "These results demonstrate the need for improved persistence with GLP-1 agonist treatment."
She noted that other investigators have estimated that reducing the rate of nonadherence to antidiabetes medications could avoid 700,000 emergency department visits and 341,000 hospitalizations annually, with a resultant $4.7 billion annual savings in health care costs (Health Aff. [Millwood] 2012;31:1836-46).
GlaxoSmithKline funded Dr. Koro’s study. The company’s investigational GLP-1 agonist albiglutide is under review by the Food and Drug Administration.
CHICAGO – Only 31% of patients with type 2 diabetes who were placed on a glucagonlike peptide-1 agonist persisted on the medication for at least 6 months in a very large national retrospective cohort study.
That’s significantly lower than the 39% rate of 6-month treatment persistence in patients placed on a dipeptidyl-peptidase-4 inhibitor, Carol E. Koro, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
The 12-month treatment discontinuation rate was 89% in patients placed on a GLP-1 agonist, compared with 82% for patients on a DPP-4 inhibitor and 84% among those placed on other antidiabetic agents, added Dr. Koro, an epidemiologist at GlaxoSmithKline in Research Triangle Park, N.C.
She presented a retrospective study examining treatment utilization patterns among 134,696 commercially insured type 2 diabetes patients placed on a GLP-1 agonist, 202,269 given a DPP-4 inhibitor, and 1,014,630 on other antidiabetic agents. The data source was the Truven Health Analytics MarketScan database for 2005-2011.
Nonadherence was defined by a prescription refill pattern indicating that less than 80% of a prescribed medication was being taken. The nonadherence rate was 9.6% in patients whose physicians prescribed a GLP-1 agonist, 5.4% in those placed on a DPP-4 inhibitor, and 4.4% in patients on other antidiabetic agents.
The median time to treatment discontinuation was 90 days in the GLP-1 agonist cohort, 120 days for the DPP-4 inhibitor cohort, and 90 days for patients assigned to other antidiabetic agents. The mean time to discontinuation was 178 days for the GLP-1 agonist group, 226 days for the DPP-4 inhibitor cohort, and 196 days for the group on other antidiabetic medications.
This was not a randomized trial, and the GLP-1 agonist cohort differed from the other two groups in important ways.
For example, they were more likely to be female: 57% of the GLP-1 agonist cohort were women, compared with 44% in the DPP-4 inhibitor cohort and 47% of patients on other antidiabetic agents. The GLP-1 agonist group also had a greater prevalence of obesity: 12%, compared with 8% in the DPP-4 inhibitor cohort and a similar figure among those on other antidiabetic agents. In addition, the GLP-1 agonist cohort had a higher baseline prevalence of microvascular complications.
Only 8% of patients placed on a GLP-1 agonist initiated the drug as new therapy; 78% started it as add-on therapy. In contrast, 17% of patients initiated a DPP-4 inhibitor as new therapy, while 59% employed the drug as add-on therapy.
The subgroup of the GLP-1 agonist cohort who initiated the drug as an add-on to basal insulin had a higher baseline prevalence of microvascular complications and greater use of antilipidemic drugs, antihypertensive agents, and antiplatelet agents than did those using the drug as an add-on to oral antidiabetic therapy. Nevertheless, the 6-month treatment persistence rates in the two subgroups were nearly identical.
The take-away message from this study is clear, Dr. Koro said: "These results demonstrate the need for improved persistence with GLP-1 agonist treatment."
She noted that other investigators have estimated that reducing the rate of nonadherence to antidiabetes medications could avoid 700,000 emergency department visits and 341,000 hospitalizations annually, with a resultant $4.7 billion annual savings in health care costs (Health Aff. [Millwood] 2012;31:1836-46).
GlaxoSmithKline funded Dr. Koro’s study. The company’s investigational GLP-1 agonist albiglutide is under review by the Food and Drug Administration.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Twelve months after a very large group of type 2 diabetes patients were placed on a GLP-1 agonist, 89% had discontinued the drug, a significantly higher rate than the 82% treatment discontinuation rate in patients placed on a DPP-4 inhibitor and the 84% rate among patients started on other antidiabetic agents.
Data source: This was a retrospective cohort study that included more than 1.35 million commercially insured patients with type 2 diabetes.
Disclosures: GlaxoSmithKline funded the study. Dr. Koro is an employee of GlaxoSmithKline.
Glucose management program reduced hospital costs
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Hospital costs were reduced by $3,000 per admission during a glucose management program.
Data source: A retrospective cohort study of 16,537 unique admissions that were nonpregnant adults who were not in critical care units and had a diagnosis code for diabetes or hyperglycemia between January 2006 and December 2009.
Disclosures: Dr. Spanakis reported having no financial conflicts of interest.
Statins for low CVD risk? Check glucose first
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
AT ADA 2013
Major finding: In patients with a 5-year CVD risk of 5% and normal glucose tolerance, statins were not cost effective ($101,800 per quality-adjusted life-year).
Data source: The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
Disclosures: Dr. Zhang and Dr. Zhou reported having no relevant financial disclosures.
Weight-loss program for veterans cut diabetes risk
CHICAGO – A low-cost lifestyle intervention program designed for real-world application in obese and overweight patients achieved sustained weight loss and a reduced risk of developing diabetes in a large 3-year observational study.
The MOVE! program (Managing Overweight and/or Obesity in Veterans Everywhere) was launched in 2005 in 130 hospitals and clinics in the Department of Veterans Affairs (VA) system, the nation’s largest integrated health care system. MOVE! is based upon principles proven effective in the National Institutes of Health’s landmark Diabetes Prevention Program (DPP), an intensive diet and exercise program that achieved a sustained 34% reduction in the incidence of diabetes at 10 years.
The problem, according to Sandra L. Jackson, is that the DPP and other successful research projects use patients who volunteer to participate and thus may be particularly highly motivated.
"We know little about the results that can be achieved in real-world health care settings, where participants are patients and their health care providers recommend a change in lifestyle," she noted in presenting the MOVE! results at the annual scientific sessions of the American Diabetes Association.
That was the impetus for her study of 3-year outcomes nationally in MOVE! The results were so impressive that MOVE! was named one of the five studies selected for an encore presentation at the ADA President’s Oral Session out of the more than 2,000 studies presented at the conference.
The MOVE! program consists of 8-12 weekly group sessions focused primarily on physical activity and nutrition. More than 400,000 veterans have participated in MOVE! since 2005. They signed up for one of two reasons: either they were obese, or they were overweight with a weight-related chronic health condition, such as osteoarthritis, coronary artery disease, diabetes, or sleep apnea. At their first MOVE! session, 38% of participants were known to have diabetes.
Ms. Jackson, a PhD candidate at Emory University, Atlanta, reported on the 135,686 MOVE! participants with 3-year follow-up data, comparing their outcomes with roughly 1.5 million VA patients who were MOVE! eligible but chose not to participate.
A total of 8.7% of participants were classified as intense and sustained in their involvement with the program based upon their having attended at least eight sessions within a 6-month period, with at least 129 days between the first and last session. Everyone else was categorized as "less involved."
Among the overall group of nearly 136,000 patients, mean body mass index dropped over the course of 3 years of follow-up from 36.3 kg/m2 to 35.8 kg/m2, representing a 1.3% loss in body weight. The intense and sustained participants lost an average of 2.5% of their initial body weight, compared with a 1% loss in the less-involved subjects.
The active participants typically experienced virtually all of their weight loss during the first 6 months, then maintained their new body weight for the next 2.5 years.
Overall, three-quarters of the intense and sustained participants lost any weight or maintained their baseline body weight over 3 years. In contrast, two-thirds of the less-active participants did so.
Diabetes risk moves down
In a multivariate analysis adjusted for baseline BMI, age, sex, and the use of medications that affect body weight, patients who lost any weight or remained weight stable over 3 years were 16% less likely to develop new-onset diabetes than those who gained weight.
The intense and sustained MOVE! participants were significantly more likely to experience a clinically meaningful weight loss of 5%. A total of 28% of them did so, compared with 16% of the less-active participants and 11% of MOVE!-eligible nonparticipants.
MOVE! enrollees with diabetes at baseline were more likely to become intense and sustained participants than those without baseline diabetes, by a margin of 9.6% to 7.8%. Overall, patients with diabetes also lost more weight: a mean of 1.7% body weight at 3 years, compared with a 0.9% drop in nondiabetic participants.
Among the 66,933 MOVE! participants without diabetes at baseline, the 3-year incidence of diabetes was 18.7%. A progressive relationship existed between weight change and diabetes incidence. At the extremes, participants who lost at least 10% of their initial body weight had a 3-year incidence of diabetes of 15%, while those with a 10% or greater weight gain had a 22% incidence of diabetes.
In a multivariate analysis, the intense and sustained participants in MOVE! had a 33% reduction in incident diabetes over 3 years, compared with the roughly 1.5 million VA patients who were MOVE! eligible but didn’t participate.
Ms. Jackson noted that a major limitation of the MOVE! program is that less than 10% of participants are actively involved. Those are the ones who reap the greatest benefits in terms of weight loss and reduced risk of diabetes.
"We need to learn how to encourage participation," she observed.
MOVE! to more health plans?
Ms. Jackson and her coinvestigators see MOVE! as well suited for adoption by other large national health care organizations.
MOVE! differs from the DPP in several key ways. It’s shorter, with 8-12 weekly group sessions largely devoted to nutrition and physical activity, compared with 16 sessions in DPP. The MOVE! classes can be taken in any order, while the DPP program requires sessions to be done in a specific sequence. MOVE! sessions can be run by exercise physiologists, nutritionists, diabetes educators, and other professionals; DPP uses a single coach.
Also, MOVE! is less structured than the DPP in that MOVE! emphasizes individualized, patient-determined goal setting developed through motivational interviewing techniques, while the DPP features fixed, generic goals.
For example, whereas the DPP set a target of 150 minutes of moderate exercise per week, MOVE! is more inclusive. It is open to veterans for whom that exercise goal may not be achievable.
Another important difference: Eligibility for MOVE! is based upon body weight, and many participants already have diabetes. In contrast, DPP participants had to be prediabetic, Ms. Jackson noted.
Future MOVE! analyses will explore the program’s impact upon participants’ health and resource utilization.
The originality of the MOVE! program is that it allows people with or without diabetes to participate, explained session chair Dr. Elbert S. Huang of the department of medicine at the University of Chicago. In contrast, most of the classic diabetes prevention studies had very narrow entry criteria. How is it possible, he asked, for such a wide range of patients in a given class to stay on the same page in terms of goal setting?
"As a practical matter," Ms. Jackson explained, "it’s much easier for the VA system to allow all comers who are obese or overweight with a weight-related health condition to participate. The goals are individualized. The program uses the principles of motivational interviewing to ask veterans, ‘How do you want to change your life?’ "
The Department of Veterans Affairs supported the study. Ms. Jackson reported having no conflicts of interest.
CHICAGO – A low-cost lifestyle intervention program designed for real-world application in obese and overweight patients achieved sustained weight loss and a reduced risk of developing diabetes in a large 3-year observational study.
The MOVE! program (Managing Overweight and/or Obesity in Veterans Everywhere) was launched in 2005 in 130 hospitals and clinics in the Department of Veterans Affairs (VA) system, the nation’s largest integrated health care system. MOVE! is based upon principles proven effective in the National Institutes of Health’s landmark Diabetes Prevention Program (DPP), an intensive diet and exercise program that achieved a sustained 34% reduction in the incidence of diabetes at 10 years.
The problem, according to Sandra L. Jackson, is that the DPP and other successful research projects use patients who volunteer to participate and thus may be particularly highly motivated.
"We know little about the results that can be achieved in real-world health care settings, where participants are patients and their health care providers recommend a change in lifestyle," she noted in presenting the MOVE! results at the annual scientific sessions of the American Diabetes Association.
That was the impetus for her study of 3-year outcomes nationally in MOVE! The results were so impressive that MOVE! was named one of the five studies selected for an encore presentation at the ADA President’s Oral Session out of the more than 2,000 studies presented at the conference.
The MOVE! program consists of 8-12 weekly group sessions focused primarily on physical activity and nutrition. More than 400,000 veterans have participated in MOVE! since 2005. They signed up for one of two reasons: either they were obese, or they were overweight with a weight-related chronic health condition, such as osteoarthritis, coronary artery disease, diabetes, or sleep apnea. At their first MOVE! session, 38% of participants were known to have diabetes.
Ms. Jackson, a PhD candidate at Emory University, Atlanta, reported on the 135,686 MOVE! participants with 3-year follow-up data, comparing their outcomes with roughly 1.5 million VA patients who were MOVE! eligible but chose not to participate.
A total of 8.7% of participants were classified as intense and sustained in their involvement with the program based upon their having attended at least eight sessions within a 6-month period, with at least 129 days between the first and last session. Everyone else was categorized as "less involved."
Among the overall group of nearly 136,000 patients, mean body mass index dropped over the course of 3 years of follow-up from 36.3 kg/m2 to 35.8 kg/m2, representing a 1.3% loss in body weight. The intense and sustained participants lost an average of 2.5% of their initial body weight, compared with a 1% loss in the less-involved subjects.
The active participants typically experienced virtually all of their weight loss during the first 6 months, then maintained their new body weight for the next 2.5 years.
Overall, three-quarters of the intense and sustained participants lost any weight or maintained their baseline body weight over 3 years. In contrast, two-thirds of the less-active participants did so.
Diabetes risk moves down
In a multivariate analysis adjusted for baseline BMI, age, sex, and the use of medications that affect body weight, patients who lost any weight or remained weight stable over 3 years were 16% less likely to develop new-onset diabetes than those who gained weight.
The intense and sustained MOVE! participants were significantly more likely to experience a clinically meaningful weight loss of 5%. A total of 28% of them did so, compared with 16% of the less-active participants and 11% of MOVE!-eligible nonparticipants.
MOVE! enrollees with diabetes at baseline were more likely to become intense and sustained participants than those without baseline diabetes, by a margin of 9.6% to 7.8%. Overall, patients with diabetes also lost more weight: a mean of 1.7% body weight at 3 years, compared with a 0.9% drop in nondiabetic participants.
Among the 66,933 MOVE! participants without diabetes at baseline, the 3-year incidence of diabetes was 18.7%. A progressive relationship existed between weight change and diabetes incidence. At the extremes, participants who lost at least 10% of their initial body weight had a 3-year incidence of diabetes of 15%, while those with a 10% or greater weight gain had a 22% incidence of diabetes.
In a multivariate analysis, the intense and sustained participants in MOVE! had a 33% reduction in incident diabetes over 3 years, compared with the roughly 1.5 million VA patients who were MOVE! eligible but didn’t participate.
Ms. Jackson noted that a major limitation of the MOVE! program is that less than 10% of participants are actively involved. Those are the ones who reap the greatest benefits in terms of weight loss and reduced risk of diabetes.
"We need to learn how to encourage participation," she observed.
MOVE! to more health plans?
Ms. Jackson and her coinvestigators see MOVE! as well suited for adoption by other large national health care organizations.
MOVE! differs from the DPP in several key ways. It’s shorter, with 8-12 weekly group sessions largely devoted to nutrition and physical activity, compared with 16 sessions in DPP. The MOVE! classes can be taken in any order, while the DPP program requires sessions to be done in a specific sequence. MOVE! sessions can be run by exercise physiologists, nutritionists, diabetes educators, and other professionals; DPP uses a single coach.
Also, MOVE! is less structured than the DPP in that MOVE! emphasizes individualized, patient-determined goal setting developed through motivational interviewing techniques, while the DPP features fixed, generic goals.
For example, whereas the DPP set a target of 150 minutes of moderate exercise per week, MOVE! is more inclusive. It is open to veterans for whom that exercise goal may not be achievable.
Another important difference: Eligibility for MOVE! is based upon body weight, and many participants already have diabetes. In contrast, DPP participants had to be prediabetic, Ms. Jackson noted.
Future MOVE! analyses will explore the program’s impact upon participants’ health and resource utilization.
The originality of the MOVE! program is that it allows people with or without diabetes to participate, explained session chair Dr. Elbert S. Huang of the department of medicine at the University of Chicago. In contrast, most of the classic diabetes prevention studies had very narrow entry criteria. How is it possible, he asked, for such a wide range of patients in a given class to stay on the same page in terms of goal setting?
"As a practical matter," Ms. Jackson explained, "it’s much easier for the VA system to allow all comers who are obese or overweight with a weight-related health condition to participate. The goals are individualized. The program uses the principles of motivational interviewing to ask veterans, ‘How do you want to change your life?’ "
The Department of Veterans Affairs supported the study. Ms. Jackson reported having no conflicts of interest.
CHICAGO – A low-cost lifestyle intervention program designed for real-world application in obese and overweight patients achieved sustained weight loss and a reduced risk of developing diabetes in a large 3-year observational study.
The MOVE! program (Managing Overweight and/or Obesity in Veterans Everywhere) was launched in 2005 in 130 hospitals and clinics in the Department of Veterans Affairs (VA) system, the nation’s largest integrated health care system. MOVE! is based upon principles proven effective in the National Institutes of Health’s landmark Diabetes Prevention Program (DPP), an intensive diet and exercise program that achieved a sustained 34% reduction in the incidence of diabetes at 10 years.
The problem, according to Sandra L. Jackson, is that the DPP and other successful research projects use patients who volunteer to participate and thus may be particularly highly motivated.
"We know little about the results that can be achieved in real-world health care settings, where participants are patients and their health care providers recommend a change in lifestyle," she noted in presenting the MOVE! results at the annual scientific sessions of the American Diabetes Association.
That was the impetus for her study of 3-year outcomes nationally in MOVE! The results were so impressive that MOVE! was named one of the five studies selected for an encore presentation at the ADA President’s Oral Session out of the more than 2,000 studies presented at the conference.
The MOVE! program consists of 8-12 weekly group sessions focused primarily on physical activity and nutrition. More than 400,000 veterans have participated in MOVE! since 2005. They signed up for one of two reasons: either they were obese, or they were overweight with a weight-related chronic health condition, such as osteoarthritis, coronary artery disease, diabetes, or sleep apnea. At their first MOVE! session, 38% of participants were known to have diabetes.
Ms. Jackson, a PhD candidate at Emory University, Atlanta, reported on the 135,686 MOVE! participants with 3-year follow-up data, comparing their outcomes with roughly 1.5 million VA patients who were MOVE! eligible but chose not to participate.
A total of 8.7% of participants were classified as intense and sustained in their involvement with the program based upon their having attended at least eight sessions within a 6-month period, with at least 129 days between the first and last session. Everyone else was categorized as "less involved."
Among the overall group of nearly 136,000 patients, mean body mass index dropped over the course of 3 years of follow-up from 36.3 kg/m2 to 35.8 kg/m2, representing a 1.3% loss in body weight. The intense and sustained participants lost an average of 2.5% of their initial body weight, compared with a 1% loss in the less-involved subjects.
The active participants typically experienced virtually all of their weight loss during the first 6 months, then maintained their new body weight for the next 2.5 years.
Overall, three-quarters of the intense and sustained participants lost any weight or maintained their baseline body weight over 3 years. In contrast, two-thirds of the less-active participants did so.
Diabetes risk moves down
In a multivariate analysis adjusted for baseline BMI, age, sex, and the use of medications that affect body weight, patients who lost any weight or remained weight stable over 3 years were 16% less likely to develop new-onset diabetes than those who gained weight.
The intense and sustained MOVE! participants were significantly more likely to experience a clinically meaningful weight loss of 5%. A total of 28% of them did so, compared with 16% of the less-active participants and 11% of MOVE!-eligible nonparticipants.
MOVE! enrollees with diabetes at baseline were more likely to become intense and sustained participants than those without baseline diabetes, by a margin of 9.6% to 7.8%. Overall, patients with diabetes also lost more weight: a mean of 1.7% body weight at 3 years, compared with a 0.9% drop in nondiabetic participants.
Among the 66,933 MOVE! participants without diabetes at baseline, the 3-year incidence of diabetes was 18.7%. A progressive relationship existed between weight change and diabetes incidence. At the extremes, participants who lost at least 10% of their initial body weight had a 3-year incidence of diabetes of 15%, while those with a 10% or greater weight gain had a 22% incidence of diabetes.
In a multivariate analysis, the intense and sustained participants in MOVE! had a 33% reduction in incident diabetes over 3 years, compared with the roughly 1.5 million VA patients who were MOVE! eligible but didn’t participate.
Ms. Jackson noted that a major limitation of the MOVE! program is that less than 10% of participants are actively involved. Those are the ones who reap the greatest benefits in terms of weight loss and reduced risk of diabetes.
"We need to learn how to encourage participation," she observed.
MOVE! to more health plans?
Ms. Jackson and her coinvestigators see MOVE! as well suited for adoption by other large national health care organizations.
MOVE! differs from the DPP in several key ways. It’s shorter, with 8-12 weekly group sessions largely devoted to nutrition and physical activity, compared with 16 sessions in DPP. The MOVE! classes can be taken in any order, while the DPP program requires sessions to be done in a specific sequence. MOVE! sessions can be run by exercise physiologists, nutritionists, diabetes educators, and other professionals; DPP uses a single coach.
Also, MOVE! is less structured than the DPP in that MOVE! emphasizes individualized, patient-determined goal setting developed through motivational interviewing techniques, while the DPP features fixed, generic goals.
For example, whereas the DPP set a target of 150 minutes of moderate exercise per week, MOVE! is more inclusive. It is open to veterans for whom that exercise goal may not be achievable.
Another important difference: Eligibility for MOVE! is based upon body weight, and many participants already have diabetes. In contrast, DPP participants had to be prediabetic, Ms. Jackson noted.
Future MOVE! analyses will explore the program’s impact upon participants’ health and resource utilization.
The originality of the MOVE! program is that it allows people with or without diabetes to participate, explained session chair Dr. Elbert S. Huang of the department of medicine at the University of Chicago. In contrast, most of the classic diabetes prevention studies had very narrow entry criteria. How is it possible, he asked, for such a wide range of patients in a given class to stay on the same page in terms of goal setting?
"As a practical matter," Ms. Jackson explained, "it’s much easier for the VA system to allow all comers who are obese or overweight with a weight-related health condition to participate. The goals are individualized. The program uses the principles of motivational interviewing to ask veterans, ‘How do you want to change your life?’ "
The Department of Veterans Affairs supported the study. Ms. Jackson reported having no conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Twenty-eight percent of heavy patients who were active participants in a novel lifestyle change program experienced a clinically meaningful weight loss of at least 5% at 3 years, compared with 16% who were less actively engaged in the program and 11% of patients who were eligible for the program but chose not to participate.
Data source: This was a retrospective, observational study involving 135,686 military veterans who took their health care providers’ recommendation to enroll in the lifestyle change program, and 1.5 million other patients who were candidates for the program but elected not to participate.
Disclosures: The U.S. Department of Veterans Affairs supported the study. The presenter reported having no financial conflicts.