User login
American Diabetes Association (ADA): Annual Scientific Sessions
Overtreatment common in high-risk diabetes patients
CHICAGO – Glycemic overtreatment of high-risk diabetic patients is rampant within the Veterans Affairs health care system, according to a national study.
Moreover, because many of these diabetic veterans who are at high risk for serious hypoglycemia are also Medicare eligible, it’s quite likely that overtreatment is a common problem in the Medicare population as well.
"I think these findings are directly relevant to Medicare," Dr. Leonard M. Pogach said in presenting the VA study results at the annual scientific sessions of the American Diabetes Association.
Current performance measures do not assess potential overtreatment of high-risk diabetic patients in either the VA or Medicare populations. But as a result of the VA study findings, joint federal efforts are underway to address this shortcoming, according to Dr. Pogach, who is national program director for endocrinology and diabetes at the Veterans Health Administration and professor of medicine at the New Jersey Medical School, Newark.
He cited as a major impetus for the VA study an eye-opening 2011 report by investigators at the Centers for Disease Control and Prevention that identified insulin and sulfonylurea drugs as the combined number-two cause of emergency hospitalizations for adverse drug events in the United States, second only to warfarin (N. Engl. J. Med. 2011;365:2002-12).
As part of the Choosing Wisely campaign, the American Geriatrics Society recommends that medications other than metformin not be routinely used to lower hemoglobin A1c below 7.5% in patients older than 65 years. The group further recommends an HbA1c target of 8%-9% for those with serious comorbid conditions.
Similarly, the ADA now recommends an HbA1c target of 7.5%-8.0% or slightly more in patients at increased risk for serious hypoglycemia or with reduced life expectancy, rather than its former universal goal of less than 7.0%.
For purposes of the VA study, Dr. Pogach and coinvestigators defined "high-risk" diabetes patients as those receiving insulin and/or sulfonylurea therapy and who are at least 70 years of age, have renal impairment as reflected in a serum creatinine level greater than 1.7 mg/dL, or have been diagnosed with cognitive impairment or dementia.
During the study year of 2009, a total of 285,476 of 652,738 VA patients, or 44%,with diabetes on insulin and/or sulfonylurea therapy qualified as high risk, based upon that definition. They received treatment in 139 VA facilities in 21 regions nationally.
Overall, 48% of these high-risk diabetes patients were likely being overtreated, as reflected in an HbA1c below 7.0%. Moreover, one-quarter of the high-risk group had an HbA1c below 6.5%, and 10% had an HbA1c of less than 6.0%, Dr. Pogach reported.
He added that these results likely underestimate the true extent of the glycemic overtreatment problem, because he and his coinvestigators defined "high risk" quite conservatively – based simply upon advanced age, renal dysfunction, and cognitive impairment.
Had they included other reasonable criteria – specifically, diminished life expectancy; stroke and other major neurologic disorders; cardiovascular disease; major depression; alcohol and/or drug abuse; and advanced diabetic complications – the proportion of the 652,738 diabetic VA patients on insulin and/or sulfonylurea therapy who would have qualified as being at high risk for serious hypoglycemia would have climbed from 44% all the way up to 71%.
The variation in overtreatment rates from region to region within the VA system was fairly tight. The range was much greater among facilities within a given regional district, where overtreatment rates varied from a low of 37% at a select few facilities to as high as 63%.
"We were able to identify several facilities with statistically remarkably lower rates of overtreatment than in the larger regions they lie in," Dr. Pogach noted. "Those are the sites where we might want to do site visits and qualitative studies to try to figure out what made them different. What is it about their culture or their patients? What happened in those places that we might subsequently want to replicate?"
Of note, the VA never adopted the one-size-fits-all goal of an HbA1c below 7.0% that the ADA recommended until recently. For more than a decade, VA clinical practice guidelines have included stratified glycemic targets based on comorbidities and life expectancy.
One audience member took issue with Dr. Pogach’s use of the word "overtreatment."
"Overtreatment is really a somewhat pejorative term," he argued. "I think you really have to have data showing that we are harming these people, not in terms of the surrogate outcome of hypoglycemia, but in real negative consequences."
Dr. Pogach was quick to rebut.
"I think the published results of the ACCORD and ADVANCE studies demonstrate that there’s a very strong association between cardiovascular morbidity and mortality and self-reported hypoglycemia, with adjusted odds ratios of about two and one-half," he explained. "I don’t think those data will ever be beat. We’re never going to have a randomized trial. So, I think the association with adverse outcomes is very clear, although we don’t know if hypoglycemia is the proximate cause or not."
The Department of Veterans Affairs supported the study. Dr. Pogach reported having no financial conflicts of interest.
CHICAGO – Glycemic overtreatment of high-risk diabetic patients is rampant within the Veterans Affairs health care system, according to a national study.
Moreover, because many of these diabetic veterans who are at high risk for serious hypoglycemia are also Medicare eligible, it’s quite likely that overtreatment is a common problem in the Medicare population as well.
"I think these findings are directly relevant to Medicare," Dr. Leonard M. Pogach said in presenting the VA study results at the annual scientific sessions of the American Diabetes Association.
Current performance measures do not assess potential overtreatment of high-risk diabetic patients in either the VA or Medicare populations. But as a result of the VA study findings, joint federal efforts are underway to address this shortcoming, according to Dr. Pogach, who is national program director for endocrinology and diabetes at the Veterans Health Administration and professor of medicine at the New Jersey Medical School, Newark.
He cited as a major impetus for the VA study an eye-opening 2011 report by investigators at the Centers for Disease Control and Prevention that identified insulin and sulfonylurea drugs as the combined number-two cause of emergency hospitalizations for adverse drug events in the United States, second only to warfarin (N. Engl. J. Med. 2011;365:2002-12).
As part of the Choosing Wisely campaign, the American Geriatrics Society recommends that medications other than metformin not be routinely used to lower hemoglobin A1c below 7.5% in patients older than 65 years. The group further recommends an HbA1c target of 8%-9% for those with serious comorbid conditions.
Similarly, the ADA now recommends an HbA1c target of 7.5%-8.0% or slightly more in patients at increased risk for serious hypoglycemia or with reduced life expectancy, rather than its former universal goal of less than 7.0%.
For purposes of the VA study, Dr. Pogach and coinvestigators defined "high-risk" diabetes patients as those receiving insulin and/or sulfonylurea therapy and who are at least 70 years of age, have renal impairment as reflected in a serum creatinine level greater than 1.7 mg/dL, or have been diagnosed with cognitive impairment or dementia.
During the study year of 2009, a total of 285,476 of 652,738 VA patients, or 44%,with diabetes on insulin and/or sulfonylurea therapy qualified as high risk, based upon that definition. They received treatment in 139 VA facilities in 21 regions nationally.
Overall, 48% of these high-risk diabetes patients were likely being overtreated, as reflected in an HbA1c below 7.0%. Moreover, one-quarter of the high-risk group had an HbA1c below 6.5%, and 10% had an HbA1c of less than 6.0%, Dr. Pogach reported.
He added that these results likely underestimate the true extent of the glycemic overtreatment problem, because he and his coinvestigators defined "high risk" quite conservatively – based simply upon advanced age, renal dysfunction, and cognitive impairment.
Had they included other reasonable criteria – specifically, diminished life expectancy; stroke and other major neurologic disorders; cardiovascular disease; major depression; alcohol and/or drug abuse; and advanced diabetic complications – the proportion of the 652,738 diabetic VA patients on insulin and/or sulfonylurea therapy who would have qualified as being at high risk for serious hypoglycemia would have climbed from 44% all the way up to 71%.
The variation in overtreatment rates from region to region within the VA system was fairly tight. The range was much greater among facilities within a given regional district, where overtreatment rates varied from a low of 37% at a select few facilities to as high as 63%.
"We were able to identify several facilities with statistically remarkably lower rates of overtreatment than in the larger regions they lie in," Dr. Pogach noted. "Those are the sites where we might want to do site visits and qualitative studies to try to figure out what made them different. What is it about their culture or their patients? What happened in those places that we might subsequently want to replicate?"
Of note, the VA never adopted the one-size-fits-all goal of an HbA1c below 7.0% that the ADA recommended until recently. For more than a decade, VA clinical practice guidelines have included stratified glycemic targets based on comorbidities and life expectancy.
One audience member took issue with Dr. Pogach’s use of the word "overtreatment."
"Overtreatment is really a somewhat pejorative term," he argued. "I think you really have to have data showing that we are harming these people, not in terms of the surrogate outcome of hypoglycemia, but in real negative consequences."
Dr. Pogach was quick to rebut.
"I think the published results of the ACCORD and ADVANCE studies demonstrate that there’s a very strong association between cardiovascular morbidity and mortality and self-reported hypoglycemia, with adjusted odds ratios of about two and one-half," he explained. "I don’t think those data will ever be beat. We’re never going to have a randomized trial. So, I think the association with adverse outcomes is very clear, although we don’t know if hypoglycemia is the proximate cause or not."
The Department of Veterans Affairs supported the study. Dr. Pogach reported having no financial conflicts of interest.
CHICAGO – Glycemic overtreatment of high-risk diabetic patients is rampant within the Veterans Affairs health care system, according to a national study.
Moreover, because many of these diabetic veterans who are at high risk for serious hypoglycemia are also Medicare eligible, it’s quite likely that overtreatment is a common problem in the Medicare population as well.
"I think these findings are directly relevant to Medicare," Dr. Leonard M. Pogach said in presenting the VA study results at the annual scientific sessions of the American Diabetes Association.
Current performance measures do not assess potential overtreatment of high-risk diabetic patients in either the VA or Medicare populations. But as a result of the VA study findings, joint federal efforts are underway to address this shortcoming, according to Dr. Pogach, who is national program director for endocrinology and diabetes at the Veterans Health Administration and professor of medicine at the New Jersey Medical School, Newark.
He cited as a major impetus for the VA study an eye-opening 2011 report by investigators at the Centers for Disease Control and Prevention that identified insulin and sulfonylurea drugs as the combined number-two cause of emergency hospitalizations for adverse drug events in the United States, second only to warfarin (N. Engl. J. Med. 2011;365:2002-12).
As part of the Choosing Wisely campaign, the American Geriatrics Society recommends that medications other than metformin not be routinely used to lower hemoglobin A1c below 7.5% in patients older than 65 years. The group further recommends an HbA1c target of 8%-9% for those with serious comorbid conditions.
Similarly, the ADA now recommends an HbA1c target of 7.5%-8.0% or slightly more in patients at increased risk for serious hypoglycemia or with reduced life expectancy, rather than its former universal goal of less than 7.0%.
For purposes of the VA study, Dr. Pogach and coinvestigators defined "high-risk" diabetes patients as those receiving insulin and/or sulfonylurea therapy and who are at least 70 years of age, have renal impairment as reflected in a serum creatinine level greater than 1.7 mg/dL, or have been diagnosed with cognitive impairment or dementia.
During the study year of 2009, a total of 285,476 of 652,738 VA patients, or 44%,with diabetes on insulin and/or sulfonylurea therapy qualified as high risk, based upon that definition. They received treatment in 139 VA facilities in 21 regions nationally.
Overall, 48% of these high-risk diabetes patients were likely being overtreated, as reflected in an HbA1c below 7.0%. Moreover, one-quarter of the high-risk group had an HbA1c below 6.5%, and 10% had an HbA1c of less than 6.0%, Dr. Pogach reported.
He added that these results likely underestimate the true extent of the glycemic overtreatment problem, because he and his coinvestigators defined "high risk" quite conservatively – based simply upon advanced age, renal dysfunction, and cognitive impairment.
Had they included other reasonable criteria – specifically, diminished life expectancy; stroke and other major neurologic disorders; cardiovascular disease; major depression; alcohol and/or drug abuse; and advanced diabetic complications – the proportion of the 652,738 diabetic VA patients on insulin and/or sulfonylurea therapy who would have qualified as being at high risk for serious hypoglycemia would have climbed from 44% all the way up to 71%.
The variation in overtreatment rates from region to region within the VA system was fairly tight. The range was much greater among facilities within a given regional district, where overtreatment rates varied from a low of 37% at a select few facilities to as high as 63%.
"We were able to identify several facilities with statistically remarkably lower rates of overtreatment than in the larger regions they lie in," Dr. Pogach noted. "Those are the sites where we might want to do site visits and qualitative studies to try to figure out what made them different. What is it about their culture or their patients? What happened in those places that we might subsequently want to replicate?"
Of note, the VA never adopted the one-size-fits-all goal of an HbA1c below 7.0% that the ADA recommended until recently. For more than a decade, VA clinical practice guidelines have included stratified glycemic targets based on comorbidities and life expectancy.
One audience member took issue with Dr. Pogach’s use of the word "overtreatment."
"Overtreatment is really a somewhat pejorative term," he argued. "I think you really have to have data showing that we are harming these people, not in terms of the surrogate outcome of hypoglycemia, but in real negative consequences."
Dr. Pogach was quick to rebut.
"I think the published results of the ACCORD and ADVANCE studies demonstrate that there’s a very strong association between cardiovascular morbidity and mortality and self-reported hypoglycemia, with adjusted odds ratios of about two and one-half," he explained. "I don’t think those data will ever be beat. We’re never going to have a randomized trial. So, I think the association with adverse outcomes is very clear, although we don’t know if hypoglycemia is the proximate cause or not."
The Department of Veterans Affairs supported the study. Dr. Pogach reported having no financial conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Nearly half of diabetes patients at high risk for serious hypoglycemia, based upon advanced age, renal dysfunction, or cognitive impairment, had an HbA1c below 7.0%, with potential adverse consequences.
Data source: This was a cross-sectional study of nearly 300,000 diabetes patients in the Veterans Affairs health care system deemed at high risk for serious hypoglycemia.
Disclosures: The U.S. Department of Veterans Affairs supported the study. Dr. Pogach had no financial conflicts.
Osteoprotegerin ups risk of vascular events in type 1 diabetes
CHICAGO – Serum osteoprotegerin is an independent predictor of both cardiovascular complications and peripheral vascular disease in type 1 diabetes, according to an analysis of a large prospective Finnish cohort.
Previous studies have suggested that high levels of the glycoprotein are associated with cardiovascular mortality in patients with diabetes, but the Finnish Diabetic Nephropathy (FinnDiane) study is the first to show an independent link between osteoprotegerin (OPG) and incident peripheral vascular disease. The findings come from a retrospective analysis of 1,939 patients with type 1 diabetes in the FinnDiane database.
Baseline serum OPG levels were significantly higher in the 80 patients who underwent amputation or peripheral revascularization than in those who did not (2.0 vs. 1.6 mg/L).
Increased OPG was a significant, independent risk marker for amputation or peripheral revascularization in multivariate analysis after adjustment for other associated factors (hazard ratio, 1.46), Dr. Daniel Gordin reported at the annual scientific sessions of the American Diabetes Association.
Baseline OPG levels were also significantly elevated in the 166 patients who suffered a first-ever cardiovascular event, compared with those with no event (2.2 vs. 1.6 mg/L). This association remained significant after adjustment for factors associated with cardiovascular disease and OPG concentrations.
Osteoprotegerin, a member of the inflammatory tumor necrosis factor superfamily, is increased in patients with coronary artery disease and is associated with the degree of arterial stiffness, explained Dr. Gordin of the division of nephrology, Helsinki (Finland) University Central Hospital.
A recent study reported that a high OPG level was an independent risk marker of all-cause mortality in hemodialysis patients and cardiovascular disease (Clin. Nephrol. 2013 Apr 2 [Epub ahead of print]).
In the current study, serum OPG levels were elevated only in patients with macroalbuminuria and/or moderate to severe renal disease. Patients with end-stage disease were excluded from the analysis.
Patients with established cardiovascular disease also had higher OPG concentrations, but this was attributable to an excess of patients with chronic kidney disease and eliminated after adjustment for renal function.
OPG remained independently correlated, however, after adjustment for renal function, with high C-reactive protein, a marker of systemic inflammation, and with hemoglobin A1c, according to study data simultaneously published online (Diabetes Care 2013;36:1827-33).
The investigators followed patients for an average of 10.4 years, and verified events and mortality from hospital discharge registries and the Finnish National Death Registry.
To no surprise, patients with macroalbuminuria and high levels of OPG at baseline were at the highest risk for cardiovascular disease events, while those with normoalbuminuria and low OPG levels were at lowest risk, Dr. Gordin said.
"However, patients with a normal urinary albumin excretion rate and high OPG concentrations, and microalbuminuric patients with high OPG concentrations, were at a similar risk for cardiovascular disease events, suggesting a predictive role of OPG on top of the associated covariates," he added.
Although patients with incident coronary heart disease and stroke had elevated baseline OPG levels, the association with OPG was not significant after correction for associated covariates.
Finally, OPG concentrations were significantly higher in the 566 patients who died during follow-up than in those who stayed alive (2.0 vs. 1.6 mg/L, but, again, the association between OPG and all-cause mortality lost statistical significance after adjustment.
Dr. Gordin observed that experimental data support a causal link between OPG and the development of cardiovascular complications. Thus, it’s possible that blocking the actions of OPG slows the development and progression of vascular disease in diabetes. In mice, however, complete blockage of OPG causes early onset of osteoporosis and arterial calcification, suggesting that a better target for prevention may be to augment its ligands, RANKL (receptor activator of nuclear factor-kappa B ligand) and TRAIL (tumor necrosis factor-alpha–related apoptosis-inducing ligand). Indeed, studies using TRAIL in apolipoprotein E knockout mice with type 1 diabetes have already reported promising reductions in atherogenesis, the authors noted.
Session moderator Dr. William Cefalu, chief and professor of endocrinology, diabetes, and metabolism at Louisiana State University, New Orleans, said in an interview that the results are interesting but that more information is needed, particularly on the impact of glucose control on OPG levels over time.
"HbA1c is going to vary day to day, so when you talk about one measure that predicts something over 7-10 years, what’s going to be the yearly variation because of glycemic control? That’s my question," he said.
The study was supported by grants from several Finnish foundations, Helsinki University Central Hospital, the Finnish Medical Society, and the Diabetes Research Foundation. Dr. Gordin reported having no financial disclosures.
CHICAGO – Serum osteoprotegerin is an independent predictor of both cardiovascular complications and peripheral vascular disease in type 1 diabetes, according to an analysis of a large prospective Finnish cohort.
Previous studies have suggested that high levels of the glycoprotein are associated with cardiovascular mortality in patients with diabetes, but the Finnish Diabetic Nephropathy (FinnDiane) study is the first to show an independent link between osteoprotegerin (OPG) and incident peripheral vascular disease. The findings come from a retrospective analysis of 1,939 patients with type 1 diabetes in the FinnDiane database.
Baseline serum OPG levels were significantly higher in the 80 patients who underwent amputation or peripheral revascularization than in those who did not (2.0 vs. 1.6 mg/L).
Increased OPG was a significant, independent risk marker for amputation or peripheral revascularization in multivariate analysis after adjustment for other associated factors (hazard ratio, 1.46), Dr. Daniel Gordin reported at the annual scientific sessions of the American Diabetes Association.
Baseline OPG levels were also significantly elevated in the 166 patients who suffered a first-ever cardiovascular event, compared with those with no event (2.2 vs. 1.6 mg/L). This association remained significant after adjustment for factors associated with cardiovascular disease and OPG concentrations.
Osteoprotegerin, a member of the inflammatory tumor necrosis factor superfamily, is increased in patients with coronary artery disease and is associated with the degree of arterial stiffness, explained Dr. Gordin of the division of nephrology, Helsinki (Finland) University Central Hospital.
A recent study reported that a high OPG level was an independent risk marker of all-cause mortality in hemodialysis patients and cardiovascular disease (Clin. Nephrol. 2013 Apr 2 [Epub ahead of print]).
In the current study, serum OPG levels were elevated only in patients with macroalbuminuria and/or moderate to severe renal disease. Patients with end-stage disease were excluded from the analysis.
Patients with established cardiovascular disease also had higher OPG concentrations, but this was attributable to an excess of patients with chronic kidney disease and eliminated after adjustment for renal function.
OPG remained independently correlated, however, after adjustment for renal function, with high C-reactive protein, a marker of systemic inflammation, and with hemoglobin A1c, according to study data simultaneously published online (Diabetes Care 2013;36:1827-33).
The investigators followed patients for an average of 10.4 years, and verified events and mortality from hospital discharge registries and the Finnish National Death Registry.
To no surprise, patients with macroalbuminuria and high levels of OPG at baseline were at the highest risk for cardiovascular disease events, while those with normoalbuminuria and low OPG levels were at lowest risk, Dr. Gordin said.
"However, patients with a normal urinary albumin excretion rate and high OPG concentrations, and microalbuminuric patients with high OPG concentrations, were at a similar risk for cardiovascular disease events, suggesting a predictive role of OPG on top of the associated covariates," he added.
Although patients with incident coronary heart disease and stroke had elevated baseline OPG levels, the association with OPG was not significant after correction for associated covariates.
Finally, OPG concentrations were significantly higher in the 566 patients who died during follow-up than in those who stayed alive (2.0 vs. 1.6 mg/L, but, again, the association between OPG and all-cause mortality lost statistical significance after adjustment.
Dr. Gordin observed that experimental data support a causal link between OPG and the development of cardiovascular complications. Thus, it’s possible that blocking the actions of OPG slows the development and progression of vascular disease in diabetes. In mice, however, complete blockage of OPG causes early onset of osteoporosis and arterial calcification, suggesting that a better target for prevention may be to augment its ligands, RANKL (receptor activator of nuclear factor-kappa B ligand) and TRAIL (tumor necrosis factor-alpha–related apoptosis-inducing ligand). Indeed, studies using TRAIL in apolipoprotein E knockout mice with type 1 diabetes have already reported promising reductions in atherogenesis, the authors noted.
Session moderator Dr. William Cefalu, chief and professor of endocrinology, diabetes, and metabolism at Louisiana State University, New Orleans, said in an interview that the results are interesting but that more information is needed, particularly on the impact of glucose control on OPG levels over time.
"HbA1c is going to vary day to day, so when you talk about one measure that predicts something over 7-10 years, what’s going to be the yearly variation because of glycemic control? That’s my question," he said.
The study was supported by grants from several Finnish foundations, Helsinki University Central Hospital, the Finnish Medical Society, and the Diabetes Research Foundation. Dr. Gordin reported having no financial disclosures.
CHICAGO – Serum osteoprotegerin is an independent predictor of both cardiovascular complications and peripheral vascular disease in type 1 diabetes, according to an analysis of a large prospective Finnish cohort.
Previous studies have suggested that high levels of the glycoprotein are associated with cardiovascular mortality in patients with diabetes, but the Finnish Diabetic Nephropathy (FinnDiane) study is the first to show an independent link between osteoprotegerin (OPG) and incident peripheral vascular disease. The findings come from a retrospective analysis of 1,939 patients with type 1 diabetes in the FinnDiane database.
Baseline serum OPG levels were significantly higher in the 80 patients who underwent amputation or peripheral revascularization than in those who did not (2.0 vs. 1.6 mg/L).
Increased OPG was a significant, independent risk marker for amputation or peripheral revascularization in multivariate analysis after adjustment for other associated factors (hazard ratio, 1.46), Dr. Daniel Gordin reported at the annual scientific sessions of the American Diabetes Association.
Baseline OPG levels were also significantly elevated in the 166 patients who suffered a first-ever cardiovascular event, compared with those with no event (2.2 vs. 1.6 mg/L). This association remained significant after adjustment for factors associated with cardiovascular disease and OPG concentrations.
Osteoprotegerin, a member of the inflammatory tumor necrosis factor superfamily, is increased in patients with coronary artery disease and is associated with the degree of arterial stiffness, explained Dr. Gordin of the division of nephrology, Helsinki (Finland) University Central Hospital.
A recent study reported that a high OPG level was an independent risk marker of all-cause mortality in hemodialysis patients and cardiovascular disease (Clin. Nephrol. 2013 Apr 2 [Epub ahead of print]).
In the current study, serum OPG levels were elevated only in patients with macroalbuminuria and/or moderate to severe renal disease. Patients with end-stage disease were excluded from the analysis.
Patients with established cardiovascular disease also had higher OPG concentrations, but this was attributable to an excess of patients with chronic kidney disease and eliminated after adjustment for renal function.
OPG remained independently correlated, however, after adjustment for renal function, with high C-reactive protein, a marker of systemic inflammation, and with hemoglobin A1c, according to study data simultaneously published online (Diabetes Care 2013;36:1827-33).
The investigators followed patients for an average of 10.4 years, and verified events and mortality from hospital discharge registries and the Finnish National Death Registry.
To no surprise, patients with macroalbuminuria and high levels of OPG at baseline were at the highest risk for cardiovascular disease events, while those with normoalbuminuria and low OPG levels were at lowest risk, Dr. Gordin said.
"However, patients with a normal urinary albumin excretion rate and high OPG concentrations, and microalbuminuric patients with high OPG concentrations, were at a similar risk for cardiovascular disease events, suggesting a predictive role of OPG on top of the associated covariates," he added.
Although patients with incident coronary heart disease and stroke had elevated baseline OPG levels, the association with OPG was not significant after correction for associated covariates.
Finally, OPG concentrations were significantly higher in the 566 patients who died during follow-up than in those who stayed alive (2.0 vs. 1.6 mg/L, but, again, the association between OPG and all-cause mortality lost statistical significance after adjustment.
Dr. Gordin observed that experimental data support a causal link between OPG and the development of cardiovascular complications. Thus, it’s possible that blocking the actions of OPG slows the development and progression of vascular disease in diabetes. In mice, however, complete blockage of OPG causes early onset of osteoporosis and arterial calcification, suggesting that a better target for prevention may be to augment its ligands, RANKL (receptor activator of nuclear factor-kappa B ligand) and TRAIL (tumor necrosis factor-alpha–related apoptosis-inducing ligand). Indeed, studies using TRAIL in apolipoprotein E knockout mice with type 1 diabetes have already reported promising reductions in atherogenesis, the authors noted.
Session moderator Dr. William Cefalu, chief and professor of endocrinology, diabetes, and metabolism at Louisiana State University, New Orleans, said in an interview that the results are interesting but that more information is needed, particularly on the impact of glucose control on OPG levels over time.
"HbA1c is going to vary day to day, so when you talk about one measure that predicts something over 7-10 years, what’s going to be the yearly variation because of glycemic control? That’s my question," he said.
The study was supported by grants from several Finnish foundations, Helsinki University Central Hospital, the Finnish Medical Society, and the Diabetes Research Foundation. Dr. Gordin reported having no financial disclosures.
AT THE ANNUAL ADA SCIENTIFIC SESSIONS
Major finding: Elevated osteoprotegerin levels were a significant independent risk marker for incident cardiovascular events (hazard ratio, 1.21) and amputation or peripheral revascularization (HR, 1.46).
Data source: Retrospective analysis of 1,939 patients with type 1 diabetes in the prospective nationwide Finnish Diabetic Nephropathy study.
Disclosures: The study was supported by grants from several Finnish foundations, Helsinki University Central Hospital, the Finnish Medical Society, and the Diabetes Research Foundation. Dr. Gordin reported having no financial disclosures.
Novel insulin/GLP-1 combo succeeds in phase III trial
CHICAGO – IDegLira, a fixed-ratio combination of insulin degludec and the glucagonlike peptide-1 agonist liraglutide, markedly outperformed each medication alone in a pivotal phase III clinical trial in patients with type 2 diabetes.
IDegLira combines the effects of insulin degludec and liraglutide in one daily injection, resulting in a substantial overall improvement in glycemic control with a low risk of hypoglycemia, weight gain, and GI adverse events," Dr. John B. Buse said in presenting the results of the phase III DUAL I (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) study at the annual scientific sessions of the American Diabetes Association.

The therapeutic rationale for the combination lies in the complementary characteristics of the two components. The net result is enhanced efficacy along with partial cross-cancellation of each drug’s side effects.
"Basal insulin is arguably the most powerful approach for controlling fasting blood glucose, and GLP-1 agonists have potent postprandial effects. The weight reduction associated with GLP-1 agonists could help mitigate the weight gain associated with basal insulin. The effects on alpha-cell function of GLP-agonists might reduce the hypoglycemia risk associated with basal insulin. And lastly, the process of individualized dosing that we use with basal insulin – getting the dose just right with regard to efficacy and safety – those concepts might reduce the GI side effects that are common with GLP-1 agonists," explained Dr. Buse, professor of medicine, chief of the division of endocrinology and metabolism, and executive associate dean for clinical research at the University of North Carolina, Chapel Hill.
All of these hypothesized benefits actually came to pass in DUAL I, a 26-week, international, open-label, phase III trial involving 1,663 type 2 diabetic subjects, all insulin-naive and inadequately controlled on metformin with or without pioglitazone. Eighty-three percent of patients were on metformin alone at enrollment, with the remainder on dual oral therapy.
Participants remained on their previous oral antidiabetic regimen during the 26-week study. They were randomized 2:1:1 to IDegLira, insulin degludec, or liraglutide (Victoza). Patients in the two insulin arms were started on 10 U/day of insulin degludec and titrated twice weekly in 2-unit increments, based upon their mean fasting plasma glucose. Ten units of IDegLira contain that amount of insulin degludec plus 0.36 mg of liraglutide. The maximum permitted dose of IDegLira contained 50 U of insulin degludec and 1.8 mg of liraglutide.
The primary study endpoint was reduction in hemoglobin A1c over the course of 26 weeks. Patients on IDegLira averaged a 1.91% drop to an HbA1c of 6.4%, significantly better than the 1.44% drop to 6.9% with insulin degludec and the 1.28% reduction to an HbA1c of 7.0% with liraglutide. In addition, the IDegLira group fared significantly better in terms of numerous secondary endpoints.
Particularly noteworthy, Dr. Buse continued, was the fact that fasting plasma glucose was reduced by an identical 65 mg/dL from a baseline of 165 mg/dL in the two-insulin treatment groups, even though the final mean daily dose of insulin degludec in the IDegLira group was 38 U, or 15 U/day less than in the group on insulin degludec alone. Confirmed hypoglycemic events occurred at a rate of 1.2 events over 26 weeks in the insulin degludec group and at a 32% lower rate in patients on IDegLira.
"That’s stunning in light of the fact that there was a 0.5% greater reduction in [HbA1c] in the IDegLira group," Dr. Buse observed.
Nausea was an issue for patients assigned to liraglutide. Roughly 10% of them per week reported nausea during titration, a rate that eventually fell off to about 3% per week after 14 weeks. In contrast, the proportion of patients in the IDegLira group who reported any nausea at all during the study was less than half that in the liraglutide group.
"This is likely related more to the titration program and the lower starting dose in the IDegLira group than to the final total milligrams of liraglutide," according to Dr. Buse. He noted that the final daily dose of liraglutide in the IDegLira group averaged 1.4 mg, compared with 1.8 mg in patients randomized to GLP-1 agonist monotherapy.
Earlier this year, Novo Nordisk, which is developing IDegLira, filed for marketing approval for the investigational agent from the European Medicines Agency. The company plans to file with the Food and Drug Administration later this year. However, the approval for insulin degludec was stalled after the FDA requested more cardiovascular safety data from the company in February 2013.
Session chair Julio Rosenstock of the Dallas Diabetes and Endocrine Center commented that the fixed-ratio combination is "a great concept" and hailed the phase III findings as "very, very impressive results." He wished, however, that the study had been set up blinded rather than open label.
Dr. Buse replied that a companion phase III trial, known as DUAL II,is in fact a blinded comparison. The results will be presented this fall in Barcelona at the annual meeting of the European Association for the Study of Diabetes.
Dr. Rosenstock called the reduction in hypoglycemic events in the IDegLira group particularly impressive, given the hefty decrease in HbA1c in that group. He asked for Dr. Buse’s thoughts as to the explanation.
It’s possibly in part a consequence of the IDegLira group’s lesser daily insulin dose, Dr. Buse replied, but liraglutide-induced improvement in alpha-cell function probably plays a bigger role.
He reported serving as a consultant to Novo Nordisk and more than 30 other pharmaceutical companies.
CHICAGO – IDegLira, a fixed-ratio combination of insulin degludec and the glucagonlike peptide-1 agonist liraglutide, markedly outperformed each medication alone in a pivotal phase III clinical trial in patients with type 2 diabetes.
IDegLira combines the effects of insulin degludec and liraglutide in one daily injection, resulting in a substantial overall improvement in glycemic control with a low risk of hypoglycemia, weight gain, and GI adverse events," Dr. John B. Buse said in presenting the results of the phase III DUAL I (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) study at the annual scientific sessions of the American Diabetes Association.

The therapeutic rationale for the combination lies in the complementary characteristics of the two components. The net result is enhanced efficacy along with partial cross-cancellation of each drug’s side effects.
"Basal insulin is arguably the most powerful approach for controlling fasting blood glucose, and GLP-1 agonists have potent postprandial effects. The weight reduction associated with GLP-1 agonists could help mitigate the weight gain associated with basal insulin. The effects on alpha-cell function of GLP-agonists might reduce the hypoglycemia risk associated with basal insulin. And lastly, the process of individualized dosing that we use with basal insulin – getting the dose just right with regard to efficacy and safety – those concepts might reduce the GI side effects that are common with GLP-1 agonists," explained Dr. Buse, professor of medicine, chief of the division of endocrinology and metabolism, and executive associate dean for clinical research at the University of North Carolina, Chapel Hill.
All of these hypothesized benefits actually came to pass in DUAL I, a 26-week, international, open-label, phase III trial involving 1,663 type 2 diabetic subjects, all insulin-naive and inadequately controlled on metformin with or without pioglitazone. Eighty-three percent of patients were on metformin alone at enrollment, with the remainder on dual oral therapy.
Participants remained on their previous oral antidiabetic regimen during the 26-week study. They were randomized 2:1:1 to IDegLira, insulin degludec, or liraglutide (Victoza). Patients in the two insulin arms were started on 10 U/day of insulin degludec and titrated twice weekly in 2-unit increments, based upon their mean fasting plasma glucose. Ten units of IDegLira contain that amount of insulin degludec plus 0.36 mg of liraglutide. The maximum permitted dose of IDegLira contained 50 U of insulin degludec and 1.8 mg of liraglutide.
The primary study endpoint was reduction in hemoglobin A1c over the course of 26 weeks. Patients on IDegLira averaged a 1.91% drop to an HbA1c of 6.4%, significantly better than the 1.44% drop to 6.9% with insulin degludec and the 1.28% reduction to an HbA1c of 7.0% with liraglutide. In addition, the IDegLira group fared significantly better in terms of numerous secondary endpoints.
Particularly noteworthy, Dr. Buse continued, was the fact that fasting plasma glucose was reduced by an identical 65 mg/dL from a baseline of 165 mg/dL in the two-insulin treatment groups, even though the final mean daily dose of insulin degludec in the IDegLira group was 38 U, or 15 U/day less than in the group on insulin degludec alone. Confirmed hypoglycemic events occurred at a rate of 1.2 events over 26 weeks in the insulin degludec group and at a 32% lower rate in patients on IDegLira.
"That’s stunning in light of the fact that there was a 0.5% greater reduction in [HbA1c] in the IDegLira group," Dr. Buse observed.
Nausea was an issue for patients assigned to liraglutide. Roughly 10% of them per week reported nausea during titration, a rate that eventually fell off to about 3% per week after 14 weeks. In contrast, the proportion of patients in the IDegLira group who reported any nausea at all during the study was less than half that in the liraglutide group.
"This is likely related more to the titration program and the lower starting dose in the IDegLira group than to the final total milligrams of liraglutide," according to Dr. Buse. He noted that the final daily dose of liraglutide in the IDegLira group averaged 1.4 mg, compared with 1.8 mg in patients randomized to GLP-1 agonist monotherapy.
Earlier this year, Novo Nordisk, which is developing IDegLira, filed for marketing approval for the investigational agent from the European Medicines Agency. The company plans to file with the Food and Drug Administration later this year. However, the approval for insulin degludec was stalled after the FDA requested more cardiovascular safety data from the company in February 2013.
Session chair Julio Rosenstock of the Dallas Diabetes and Endocrine Center commented that the fixed-ratio combination is "a great concept" and hailed the phase III findings as "very, very impressive results." He wished, however, that the study had been set up blinded rather than open label.
Dr. Buse replied that a companion phase III trial, known as DUAL II,is in fact a blinded comparison. The results will be presented this fall in Barcelona at the annual meeting of the European Association for the Study of Diabetes.
Dr. Rosenstock called the reduction in hypoglycemic events in the IDegLira group particularly impressive, given the hefty decrease in HbA1c in that group. He asked for Dr. Buse’s thoughts as to the explanation.
It’s possibly in part a consequence of the IDegLira group’s lesser daily insulin dose, Dr. Buse replied, but liraglutide-induced improvement in alpha-cell function probably plays a bigger role.
He reported serving as a consultant to Novo Nordisk and more than 30 other pharmaceutical companies.
CHICAGO – IDegLira, a fixed-ratio combination of insulin degludec and the glucagonlike peptide-1 agonist liraglutide, markedly outperformed each medication alone in a pivotal phase III clinical trial in patients with type 2 diabetes.
IDegLira combines the effects of insulin degludec and liraglutide in one daily injection, resulting in a substantial overall improvement in glycemic control with a low risk of hypoglycemia, weight gain, and GI adverse events," Dr. John B. Buse said in presenting the results of the phase III DUAL I (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) study at the annual scientific sessions of the American Diabetes Association.

The therapeutic rationale for the combination lies in the complementary characteristics of the two components. The net result is enhanced efficacy along with partial cross-cancellation of each drug’s side effects.
"Basal insulin is arguably the most powerful approach for controlling fasting blood glucose, and GLP-1 agonists have potent postprandial effects. The weight reduction associated with GLP-1 agonists could help mitigate the weight gain associated with basal insulin. The effects on alpha-cell function of GLP-agonists might reduce the hypoglycemia risk associated with basal insulin. And lastly, the process of individualized dosing that we use with basal insulin – getting the dose just right with regard to efficacy and safety – those concepts might reduce the GI side effects that are common with GLP-1 agonists," explained Dr. Buse, professor of medicine, chief of the division of endocrinology and metabolism, and executive associate dean for clinical research at the University of North Carolina, Chapel Hill.
All of these hypothesized benefits actually came to pass in DUAL I, a 26-week, international, open-label, phase III trial involving 1,663 type 2 diabetic subjects, all insulin-naive and inadequately controlled on metformin with or without pioglitazone. Eighty-three percent of patients were on metformin alone at enrollment, with the remainder on dual oral therapy.
Participants remained on their previous oral antidiabetic regimen during the 26-week study. They were randomized 2:1:1 to IDegLira, insulin degludec, or liraglutide (Victoza). Patients in the two insulin arms were started on 10 U/day of insulin degludec and titrated twice weekly in 2-unit increments, based upon their mean fasting plasma glucose. Ten units of IDegLira contain that amount of insulin degludec plus 0.36 mg of liraglutide. The maximum permitted dose of IDegLira contained 50 U of insulin degludec and 1.8 mg of liraglutide.
The primary study endpoint was reduction in hemoglobin A1c over the course of 26 weeks. Patients on IDegLira averaged a 1.91% drop to an HbA1c of 6.4%, significantly better than the 1.44% drop to 6.9% with insulin degludec and the 1.28% reduction to an HbA1c of 7.0% with liraglutide. In addition, the IDegLira group fared significantly better in terms of numerous secondary endpoints.
Particularly noteworthy, Dr. Buse continued, was the fact that fasting plasma glucose was reduced by an identical 65 mg/dL from a baseline of 165 mg/dL in the two-insulin treatment groups, even though the final mean daily dose of insulin degludec in the IDegLira group was 38 U, or 15 U/day less than in the group on insulin degludec alone. Confirmed hypoglycemic events occurred at a rate of 1.2 events over 26 weeks in the insulin degludec group and at a 32% lower rate in patients on IDegLira.
"That’s stunning in light of the fact that there was a 0.5% greater reduction in [HbA1c] in the IDegLira group," Dr. Buse observed.
Nausea was an issue for patients assigned to liraglutide. Roughly 10% of them per week reported nausea during titration, a rate that eventually fell off to about 3% per week after 14 weeks. In contrast, the proportion of patients in the IDegLira group who reported any nausea at all during the study was less than half that in the liraglutide group.
"This is likely related more to the titration program and the lower starting dose in the IDegLira group than to the final total milligrams of liraglutide," according to Dr. Buse. He noted that the final daily dose of liraglutide in the IDegLira group averaged 1.4 mg, compared with 1.8 mg in patients randomized to GLP-1 agonist monotherapy.
Earlier this year, Novo Nordisk, which is developing IDegLira, filed for marketing approval for the investigational agent from the European Medicines Agency. The company plans to file with the Food and Drug Administration later this year. However, the approval for insulin degludec was stalled after the FDA requested more cardiovascular safety data from the company in February 2013.
Session chair Julio Rosenstock of the Dallas Diabetes and Endocrine Center commented that the fixed-ratio combination is "a great concept" and hailed the phase III findings as "very, very impressive results." He wished, however, that the study had been set up blinded rather than open label.
Dr. Buse replied that a companion phase III trial, known as DUAL II,is in fact a blinded comparison. The results will be presented this fall in Barcelona at the annual meeting of the European Association for the Study of Diabetes.
Dr. Rosenstock called the reduction in hypoglycemic events in the IDegLira group particularly impressive, given the hefty decrease in HbA1c in that group. He asked for Dr. Buse’s thoughts as to the explanation.
It’s possibly in part a consequence of the IDegLira group’s lesser daily insulin dose, Dr. Buse replied, but liraglutide-induced improvement in alpha-cell function probably plays a bigger role.
He reported serving as a consultant to Novo Nordisk and more than 30 other pharmaceutical companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Patients with type 2 diabetes not adequately controlled on oral therapy experienced a mean 1.91% reduction in HbA1c to a final value of 6.4% in response to the addition of once-daily subcutaneous injections of IDegLira, a fixed-ratio combination of insulin degludec and liraglutide.
Data source: This was a pivotal phase III, 26-week, open-label study in which 1,663 adults with inadequately controlled type 2 diabetes on oral medication were randomized to IDegLira, insulin degludec, or liraglutide.
Disclosures: The study was sponsored by Novo Nordisk. The presenter is a consultant to the company.
Aging-related functional decline boosts diabetes risk
CHICAGO – Aging-related decline in physical functioning has emerged as a previously unrecognized independent risk factor for new-onset diabetes, according to investigators at the Centers for Disease Control and Prevention.
In a multivariate analysis involving 22,876 subjects over age 50 years at baseline in the longitudinal, prospective Health and Retirement Study, those who had mild or moderate functional decline at enrollment or who developed it during an average 8.7 years of follow-up had a 17% increased risk of subsequently being diagnosed with new-onset diabetes, compared with subjects who did not have prevalent or incident functional decline or physical disability, Barbara H. Bardenheier, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
Subjects who had severe functional decline at baseline or developed it during follow-up had a 12% excess risk of subsequent diabetes. Like mild-to-moderate functional decline, severe functional decline was also a statistically significant risk factor for later diagnosis of diabetes in a multivariate analysis adjusted for age, race, education, baseline body mass index, and socioeconomic factors. The excess diabetes risk in individuals with prevalent or incident severe functional decline would have been higher but for the fact that they also had a 2.3-fold increased risk of mortality, compared with participants without aging-related functional decline, observed Dr. Bardenheier of the CDC in Atlanta.
It has been shown consistently in longitudinal and cross-sectional studies that diabetes is associated with an increased risk of subsequent physical disability, but this analysis of the Health and Retirement Study is the first to show the converse: that aging-related functional decline and physical disability or frailty place an individual at increased risk for subsequent diabetes, she added.
The definitions of aging-related functional decline employed in this study were based on difficulty expected to last longer than 3 months in performing specific mobility measures. Five mobility measures were used: walking one block; walking several blocks; stooping, crouching, or kneeling; climbing one flight of stairs, and pushing or pulling a large object.
Mild functional decline was defined as difficulty in stooping and walking several blocks or difficulty in any two mobility measures other than stair-climbing. Moderate functional decline required either difficulty in climbing a single flight of stairs or difficulty with any three of the other mobility measures. Severe decline was defined as difficulty with at least four mobility measures.
Among the 13,143 study participants who did not have aging-related functional decline at baseline, those who developed mild functional decline during follow-up had an adjusted 19% excess risk of being diagnosed with diabetes afterward. Those who developed moderate decline had a 30% excess risk of subsequent diabetes, while those with incident severe functional decline had a 16% increased risk of diabetes along with a 2.4-fold increased mortality risk. During follow-up, 18.8% of subjects without baseline aging-related functional decline developed moderate or severe decline.
Of note, 77% of participants reported some level of functional decline during the study period. During follow-up, 15.5% of subjects developed diabetes and 25.6% of subjects died.
The clinical relevance of this new observation that aging-related functional decline is a risk factor for subsequent diabetes lies in the fact that prior studies have shown functional decline is potentially modifiable. For example, physical exercise programs geared for older patients with moderate physical frailty have been shown to be protective against further disability, according to Dr. Bardenheier.
The Health and Retirement Study is sponsored by the National Institute on Aging. Dr. Bardenheier’s analysis was funded by the CDC. She reported having no conflicts of interest.
CHICAGO – Aging-related decline in physical functioning has emerged as a previously unrecognized independent risk factor for new-onset diabetes, according to investigators at the Centers for Disease Control and Prevention.
In a multivariate analysis involving 22,876 subjects over age 50 years at baseline in the longitudinal, prospective Health and Retirement Study, those who had mild or moderate functional decline at enrollment or who developed it during an average 8.7 years of follow-up had a 17% increased risk of subsequently being diagnosed with new-onset diabetes, compared with subjects who did not have prevalent or incident functional decline or physical disability, Barbara H. Bardenheier, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
Subjects who had severe functional decline at baseline or developed it during follow-up had a 12% excess risk of subsequent diabetes. Like mild-to-moderate functional decline, severe functional decline was also a statistically significant risk factor for later diagnosis of diabetes in a multivariate analysis adjusted for age, race, education, baseline body mass index, and socioeconomic factors. The excess diabetes risk in individuals with prevalent or incident severe functional decline would have been higher but for the fact that they also had a 2.3-fold increased risk of mortality, compared with participants without aging-related functional decline, observed Dr. Bardenheier of the CDC in Atlanta.
It has been shown consistently in longitudinal and cross-sectional studies that diabetes is associated with an increased risk of subsequent physical disability, but this analysis of the Health and Retirement Study is the first to show the converse: that aging-related functional decline and physical disability or frailty place an individual at increased risk for subsequent diabetes, she added.
The definitions of aging-related functional decline employed in this study were based on difficulty expected to last longer than 3 months in performing specific mobility measures. Five mobility measures were used: walking one block; walking several blocks; stooping, crouching, or kneeling; climbing one flight of stairs, and pushing or pulling a large object.
Mild functional decline was defined as difficulty in stooping and walking several blocks or difficulty in any two mobility measures other than stair-climbing. Moderate functional decline required either difficulty in climbing a single flight of stairs or difficulty with any three of the other mobility measures. Severe decline was defined as difficulty with at least four mobility measures.
Among the 13,143 study participants who did not have aging-related functional decline at baseline, those who developed mild functional decline during follow-up had an adjusted 19% excess risk of being diagnosed with diabetes afterward. Those who developed moderate decline had a 30% excess risk of subsequent diabetes, while those with incident severe functional decline had a 16% increased risk of diabetes along with a 2.4-fold increased mortality risk. During follow-up, 18.8% of subjects without baseline aging-related functional decline developed moderate or severe decline.
Of note, 77% of participants reported some level of functional decline during the study period. During follow-up, 15.5% of subjects developed diabetes and 25.6% of subjects died.
The clinical relevance of this new observation that aging-related functional decline is a risk factor for subsequent diabetes lies in the fact that prior studies have shown functional decline is potentially modifiable. For example, physical exercise programs geared for older patients with moderate physical frailty have been shown to be protective against further disability, according to Dr. Bardenheier.
The Health and Retirement Study is sponsored by the National Institute on Aging. Dr. Bardenheier’s analysis was funded by the CDC. She reported having no conflicts of interest.
CHICAGO – Aging-related decline in physical functioning has emerged as a previously unrecognized independent risk factor for new-onset diabetes, according to investigators at the Centers for Disease Control and Prevention.
In a multivariate analysis involving 22,876 subjects over age 50 years at baseline in the longitudinal, prospective Health and Retirement Study, those who had mild or moderate functional decline at enrollment or who developed it during an average 8.7 years of follow-up had a 17% increased risk of subsequently being diagnosed with new-onset diabetes, compared with subjects who did not have prevalent or incident functional decline or physical disability, Barbara H. Bardenheier, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
Subjects who had severe functional decline at baseline or developed it during follow-up had a 12% excess risk of subsequent diabetes. Like mild-to-moderate functional decline, severe functional decline was also a statistically significant risk factor for later diagnosis of diabetes in a multivariate analysis adjusted for age, race, education, baseline body mass index, and socioeconomic factors. The excess diabetes risk in individuals with prevalent or incident severe functional decline would have been higher but for the fact that they also had a 2.3-fold increased risk of mortality, compared with participants without aging-related functional decline, observed Dr. Bardenheier of the CDC in Atlanta.
It has been shown consistently in longitudinal and cross-sectional studies that diabetes is associated with an increased risk of subsequent physical disability, but this analysis of the Health and Retirement Study is the first to show the converse: that aging-related functional decline and physical disability or frailty place an individual at increased risk for subsequent diabetes, she added.
The definitions of aging-related functional decline employed in this study were based on difficulty expected to last longer than 3 months in performing specific mobility measures. Five mobility measures were used: walking one block; walking several blocks; stooping, crouching, or kneeling; climbing one flight of stairs, and pushing or pulling a large object.
Mild functional decline was defined as difficulty in stooping and walking several blocks or difficulty in any two mobility measures other than stair-climbing. Moderate functional decline required either difficulty in climbing a single flight of stairs or difficulty with any three of the other mobility measures. Severe decline was defined as difficulty with at least four mobility measures.
Among the 13,143 study participants who did not have aging-related functional decline at baseline, those who developed mild functional decline during follow-up had an adjusted 19% excess risk of being diagnosed with diabetes afterward. Those who developed moderate decline had a 30% excess risk of subsequent diabetes, while those with incident severe functional decline had a 16% increased risk of diabetes along with a 2.4-fold increased mortality risk. During follow-up, 18.8% of subjects without baseline aging-related functional decline developed moderate or severe decline.
Of note, 77% of participants reported some level of functional decline during the study period. During follow-up, 15.5% of subjects developed diabetes and 25.6% of subjects died.
The clinical relevance of this new observation that aging-related functional decline is a risk factor for subsequent diabetes lies in the fact that prior studies have shown functional decline is potentially modifiable. For example, physical exercise programs geared for older patients with moderate physical frailty have been shown to be protective against further disability, according to Dr. Bardenheier.
The Health and Retirement Study is sponsored by the National Institute on Aging. Dr. Bardenheier’s analysis was funded by the CDC. She reported having no conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: New-onset mild aging-related decline in physical functioning resulted in a 19% excess risk of subsequently developing diabetes, while incident moderate functional decline resulted in a 30% increased risk and severe decline resulted in a 16% excess risk.
Data Source: An analysis of nearly 23,000 participants in the prospective, longitudinal Health and Retirement Study, 15.5% of whom developed diabetes during an average 8.7 years of follow-up.
Disclosures: The Health and Retirement Study is sponsored by the National Institute on Aging. This analysis was funded by the CDC. The presenter reported having no financial conflicts.
Octogenarians hard hit by insulin adverse events
CHICAGO – Older adults with diabetes are particularly vulnerable to insulin adverse event–related emergency department visits and hospitalizations, an analysis of nationally representative surveillance data has shown.
Compared with diabetes patients aged 45-64 years, those 80 years or older were 2.5 times more likely to present to the emergency department with an insulin adverse event (34.9 vs. 13.7/1,000 insulin-treated diabetes patients) and about five times more likely to be emergently hospitalized for such an event (16.1 vs. 3.3/1,000 persons).
"These data support setting glycemic targets based on risk/benefit," Dr. Andrew I. Geller of the Centers for Disease Control and Prevention said at the annual scientific sessions of the American Diabetes Association.
National guidelines are evolving in that direction. Earlier this year, the ADA recommended that older adults who are "functional, cognitively intact, and have significant life expectancy" should not have glycemic targets different from those of younger adults, but acknowledged that for some older adults, glycemic targets "might reasonably be relaxed, using individual criteria."
The American Association of Clinical Endocrinologists Diabetes Management Algorithm 2013 says that a hemoglobin A1c level "of 6.5% or less is still considered optimal if it can be achieved in a safe and affordable manner," but adds that the HbA1c target "must be individualized, based on numerous factors, such as age."
Dr. Geller suggested that reducing insulin adverse events in older adults may have a significant public health impact. Insulin is the most commonly implicated medicine in U.S. ED visits, and it’s linked to about 14,000 hospitalizations annually among adults 65 years or older. Insulins and synthetic analogues also accounted for 71% of spending growth on antidiabetes medications in 2011.
Dr. Geller and his colleagues estimated annual insulin use frequency with National Health Interview Survey data, and annual insulin adverse events with data from the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance (NEISS-CADES), an ongoing public health surveillance project based on chart abstraction, not ICD codes.
Insulin adverse events were defined as supratherapeutic effects (hypoglycemia) and therapeutic misadventures (for example, errors). Allergic reactions, local effects, and accidental exposure were excluded.
Insulin adverse events led to an estimated 97,745 emergency department visits annually from 2007 to 2011, accounting for 6.5% of all adverse drug event–related ED visits, Dr. Geller said.
While the sample had a slight predominance of males (50.4%) and a majority of whites (60.5%), there was no difference in rates among genders or races.
Patients aged 45-64 years had the highest proportion of ED visits (35%), followed by those 65-79 years (25.3%), 18-44 years (21.7%), 80 years or older (15.8%), and younger than 18 years (2.1%).
Still, patients 80 years or older had a rate of 34.9 ED visits per 1,000 diabetic persons, far surpassing rates observed among those younger than 18 years (13.7), 18-44 years (24.3), 45-64 years (13.7), and 65-79 years (16.3).
Patients aged 65-79 years had the highest proportion of hospitalizations (32.9%), but once again, those 80 years and older had the highest rate of hospitalizations per 1,000 diabetic persons (16.1), compared with the other age groups: younger than 18 years (2.5), 18-44 years (3.9), 45-64 years (3.3), and 65-79 years (6.2).
Based on clinical and laboratory findings, about 65% of patients presented with shock, loss of consciousness, or seizure (23.2%); fall or other injury (5%); and altered mental status (37%), Dr. Geller said. More than half of patients had a blood glucose level less than 50 mg/dL.
More than half (56%) of patients received intravenous dextrose or glucagon during their ED visit, while 29.3% of visits overall resulted in hospitalization, he said.
Precipitating factors were documented in about 21% of charts. The three most common were:
• Meal-related factors in 45.6%, such as a 19-year-old driver who took insulin 2 hours before hitting a tree, but had "no time to eat."
• Product mix-ups in 22%, including a 51-year-old injected by his spouse with 50 U of NovoLog instead of 50 U of Lantus.
• Incorrect dose or units in 12%, such as a 62-year-old who took 40 U of regular insulin instead of 4.
In cases where the patient took the wrong type of insulin, 52.3% of mix-ups involved a patient taking a rapid-acting agent instead of a long-acting one, Dr. Geller said.
"Perhaps the burden of insulin adverse events may be impacted by targeting at-risk individuals such as older adults, bolstering diabetes education such as meal planning, and minimizing the risk of product mix-ups, perhaps through packaging," he concluded.
Dr. Geller reported having no relevant financial disclosures.
CHICAGO – Older adults with diabetes are particularly vulnerable to insulin adverse event–related emergency department visits and hospitalizations, an analysis of nationally representative surveillance data has shown.
Compared with diabetes patients aged 45-64 years, those 80 years or older were 2.5 times more likely to present to the emergency department with an insulin adverse event (34.9 vs. 13.7/1,000 insulin-treated diabetes patients) and about five times more likely to be emergently hospitalized for such an event (16.1 vs. 3.3/1,000 persons).
"These data support setting glycemic targets based on risk/benefit," Dr. Andrew I. Geller of the Centers for Disease Control and Prevention said at the annual scientific sessions of the American Diabetes Association.
National guidelines are evolving in that direction. Earlier this year, the ADA recommended that older adults who are "functional, cognitively intact, and have significant life expectancy" should not have glycemic targets different from those of younger adults, but acknowledged that for some older adults, glycemic targets "might reasonably be relaxed, using individual criteria."
The American Association of Clinical Endocrinologists Diabetes Management Algorithm 2013 says that a hemoglobin A1c level "of 6.5% or less is still considered optimal if it can be achieved in a safe and affordable manner," but adds that the HbA1c target "must be individualized, based on numerous factors, such as age."
Dr. Geller suggested that reducing insulin adverse events in older adults may have a significant public health impact. Insulin is the most commonly implicated medicine in U.S. ED visits, and it’s linked to about 14,000 hospitalizations annually among adults 65 years or older. Insulins and synthetic analogues also accounted for 71% of spending growth on antidiabetes medications in 2011.
Dr. Geller and his colleagues estimated annual insulin use frequency with National Health Interview Survey data, and annual insulin adverse events with data from the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance (NEISS-CADES), an ongoing public health surveillance project based on chart abstraction, not ICD codes.
Insulin adverse events were defined as supratherapeutic effects (hypoglycemia) and therapeutic misadventures (for example, errors). Allergic reactions, local effects, and accidental exposure were excluded.
Insulin adverse events led to an estimated 97,745 emergency department visits annually from 2007 to 2011, accounting for 6.5% of all adverse drug event–related ED visits, Dr. Geller said.
While the sample had a slight predominance of males (50.4%) and a majority of whites (60.5%), there was no difference in rates among genders or races.
Patients aged 45-64 years had the highest proportion of ED visits (35%), followed by those 65-79 years (25.3%), 18-44 years (21.7%), 80 years or older (15.8%), and younger than 18 years (2.1%).
Still, patients 80 years or older had a rate of 34.9 ED visits per 1,000 diabetic persons, far surpassing rates observed among those younger than 18 years (13.7), 18-44 years (24.3), 45-64 years (13.7), and 65-79 years (16.3).
Patients aged 65-79 years had the highest proportion of hospitalizations (32.9%), but once again, those 80 years and older had the highest rate of hospitalizations per 1,000 diabetic persons (16.1), compared with the other age groups: younger than 18 years (2.5), 18-44 years (3.9), 45-64 years (3.3), and 65-79 years (6.2).
Based on clinical and laboratory findings, about 65% of patients presented with shock, loss of consciousness, or seizure (23.2%); fall or other injury (5%); and altered mental status (37%), Dr. Geller said. More than half of patients had a blood glucose level less than 50 mg/dL.
More than half (56%) of patients received intravenous dextrose or glucagon during their ED visit, while 29.3% of visits overall resulted in hospitalization, he said.
Precipitating factors were documented in about 21% of charts. The three most common were:
• Meal-related factors in 45.6%, such as a 19-year-old driver who took insulin 2 hours before hitting a tree, but had "no time to eat."
• Product mix-ups in 22%, including a 51-year-old injected by his spouse with 50 U of NovoLog instead of 50 U of Lantus.
• Incorrect dose or units in 12%, such as a 62-year-old who took 40 U of regular insulin instead of 4.
In cases where the patient took the wrong type of insulin, 52.3% of mix-ups involved a patient taking a rapid-acting agent instead of a long-acting one, Dr. Geller said.
"Perhaps the burden of insulin adverse events may be impacted by targeting at-risk individuals such as older adults, bolstering diabetes education such as meal planning, and minimizing the risk of product mix-ups, perhaps through packaging," he concluded.
Dr. Geller reported having no relevant financial disclosures.
CHICAGO – Older adults with diabetes are particularly vulnerable to insulin adverse event–related emergency department visits and hospitalizations, an analysis of nationally representative surveillance data has shown.
Compared with diabetes patients aged 45-64 years, those 80 years or older were 2.5 times more likely to present to the emergency department with an insulin adverse event (34.9 vs. 13.7/1,000 insulin-treated diabetes patients) and about five times more likely to be emergently hospitalized for such an event (16.1 vs. 3.3/1,000 persons).
"These data support setting glycemic targets based on risk/benefit," Dr. Andrew I. Geller of the Centers for Disease Control and Prevention said at the annual scientific sessions of the American Diabetes Association.
National guidelines are evolving in that direction. Earlier this year, the ADA recommended that older adults who are "functional, cognitively intact, and have significant life expectancy" should not have glycemic targets different from those of younger adults, but acknowledged that for some older adults, glycemic targets "might reasonably be relaxed, using individual criteria."
The American Association of Clinical Endocrinologists Diabetes Management Algorithm 2013 says that a hemoglobin A1c level "of 6.5% or less is still considered optimal if it can be achieved in a safe and affordable manner," but adds that the HbA1c target "must be individualized, based on numerous factors, such as age."
Dr. Geller suggested that reducing insulin adverse events in older adults may have a significant public health impact. Insulin is the most commonly implicated medicine in U.S. ED visits, and it’s linked to about 14,000 hospitalizations annually among adults 65 years or older. Insulins and synthetic analogues also accounted for 71% of spending growth on antidiabetes medications in 2011.
Dr. Geller and his colleagues estimated annual insulin use frequency with National Health Interview Survey data, and annual insulin adverse events with data from the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance (NEISS-CADES), an ongoing public health surveillance project based on chart abstraction, not ICD codes.
Insulin adverse events were defined as supratherapeutic effects (hypoglycemia) and therapeutic misadventures (for example, errors). Allergic reactions, local effects, and accidental exposure were excluded.
Insulin adverse events led to an estimated 97,745 emergency department visits annually from 2007 to 2011, accounting for 6.5% of all adverse drug event–related ED visits, Dr. Geller said.
While the sample had a slight predominance of males (50.4%) and a majority of whites (60.5%), there was no difference in rates among genders or races.
Patients aged 45-64 years had the highest proportion of ED visits (35%), followed by those 65-79 years (25.3%), 18-44 years (21.7%), 80 years or older (15.8%), and younger than 18 years (2.1%).
Still, patients 80 years or older had a rate of 34.9 ED visits per 1,000 diabetic persons, far surpassing rates observed among those younger than 18 years (13.7), 18-44 years (24.3), 45-64 years (13.7), and 65-79 years (16.3).
Patients aged 65-79 years had the highest proportion of hospitalizations (32.9%), but once again, those 80 years and older had the highest rate of hospitalizations per 1,000 diabetic persons (16.1), compared with the other age groups: younger than 18 years (2.5), 18-44 years (3.9), 45-64 years (3.3), and 65-79 years (6.2).
Based on clinical and laboratory findings, about 65% of patients presented with shock, loss of consciousness, or seizure (23.2%); fall or other injury (5%); and altered mental status (37%), Dr. Geller said. More than half of patients had a blood glucose level less than 50 mg/dL.
More than half (56%) of patients received intravenous dextrose or glucagon during their ED visit, while 29.3% of visits overall resulted in hospitalization, he said.
Precipitating factors were documented in about 21% of charts. The three most common were:
• Meal-related factors in 45.6%, such as a 19-year-old driver who took insulin 2 hours before hitting a tree, but had "no time to eat."
• Product mix-ups in 22%, including a 51-year-old injected by his spouse with 50 U of NovoLog instead of 50 U of Lantus.
• Incorrect dose or units in 12%, such as a 62-year-old who took 40 U of regular insulin instead of 4.
In cases where the patient took the wrong type of insulin, 52.3% of mix-ups involved a patient taking a rapid-acting agent instead of a long-acting one, Dr. Geller said.
"Perhaps the burden of insulin adverse events may be impacted by targeting at-risk individuals such as older adults, bolstering diabetes education such as meal planning, and minimizing the risk of product mix-ups, perhaps through packaging," he concluded.
Dr. Geller reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Diabetes patients aged 80 years or older were 2.5 times more likely to present to the emergency department for an insulin adverse event and five times more likely to be hospitalized than diabetes patients aged 45-64 years.
Data source: An analysis of national surveillance data from 2007 to 2011.
Disclosures: Dr. Geller reported having no relevant financial disclosures.
Case builds for counting fat, protein alongside carbs in T1DM
CHICAGO – Meals high in fat or protein cause hyperglycemia among children using intensive insulin therapy for type 1 diabetes, a study has shown.
Further, protein and fat have an additive effect on postprandial glycemic rise, based on results of the randomized study involving 33 children.
"Protein and fat should be considered in prandial insulin dose and distribution," Carmel Smart, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
In intensive insulin therapy, the mealtime insulin dose is calculated based exclusively on counting carbohydrates. A growing body of evidence, however, suggests that macronutrients other than carbohydrates influence postprandial glucose levels and insulin requirements, said Dr. Smart, a specialist diabetes dietitian at the John Hunter Children’s Hospital, Newcastle, Australia.
She highlighted a recent study showing that high-fat meals with identical carbohydrate and protein content, required more insulin than low-fat meals and, despite the additional insulin, caused more hyperglycemia in type 1 diabetics (Diabetes Care 2013;36:810-6). An early study reported that meals with added protein significantly increased late glucose responses and insulin requirements, compared with standard or fat-added meals (Am. J. Clin. Nutr. 1993;58:555-60).
For the current experiment, 33 children (aged 8-17) ate four test pancake breakfasts on different days with identical carbohydrate content (30 g), but varying protein and fat: low fat (4 g)/low protein (5 g), high fat (35 g)/low protein, low fat/high protein (40 g), and high fat/high protein. A protein supplement and double cream (50% fat) were used to vary the protein and fat content.
The meals were given after the same individually standardized insulin dose, and no activity or snacks were allowed for 5 hours after the meal. Glucose excursions or fluctuations were measured at 30-minute increments for 5 hours using continuous glucose monitoring.
At baseline, the 17 girls and 16 boys had a mean A1c level of 7.2% and a mean duration of diabetes of 4.9 years; 27 were on continuous subcutaneous insulin infusion, and 6 were on a multiple daily insulin regimen. Their average age was 12.2 years.
Compared with the low-protein/low-fat meal, postprandial glucose excursions were significantly greater at 210-300 minutes after the high-fat/low-protein meal (1.78 mmol/L vs. –0.50 mmol/L; P = .01), Dr. Smart said.
Postprandial glucose excursions also were significantly greater at 180 minutes after the high-protein/low-fat meal (2.40 mmol/L vs. 0.54 mmol/L; P = .02).
The high-fat/high-protein meal resulted in significantly higher and sustained glucose excursions from 180 to 300 minutes compared with all the other meals (P less than .04).
The effect of fat and protein was additive at all time points after 150 minutes, she said. Compared with the low-fat/low-protein meal, the average glucose excursion was 4.2 mmol/L greater at 180 minutes after the high-fat/high-protein meal and 5.4 mmol/L greater at 5 hours.
Hypoglycemia, defined by a blood glucose level of less than 3.6 mmol/L, occurred in 29 children in the 5-hour postprandial period, and was significantly different between meal types (P = .003), Dr. Smart said.
There was a significant reduction in hypoglycemia after high-protein meals (odds ratio, 0.16; P less than .0001), but no reduction after high-fat meals (OR, 0.50; P = .08).
While meals high in protein and fat cause hyperglycemia, "our results suggest that protein may have a protective effect on hypoglycemia," she said.
During a discussion of the study, an audience member thanked the investigators for conducting the study "because this is what I’ve been seeing in patients eating high-fat diets, and I haven’t known exactly what to do about their insulin when they aren’t on the pump."
Dr. Smart agreed that they’ve been seeing this phenomenon for a long time, and said clinicians can now have the confidence to tell patients that it’s not inaccurate carbohydrate counting causing the glucose fluctuations, as previously believed. Currently, the investigators are advising patients on pump therapy to use the dual-wave bolus, although novel algorithms to determine prandial glucose dosing are being refined for high-fat/high-protein diets, she added.
A study led by Kirstine Bell, Ph.D., detailing one of those algorithms, called the food insulin index, was presented during the same session, by Dr. Stephen Colagiuri.
The study was supported by grants from Australian Pediatric Endocrine Care and Hunter Children’s Research Foundation. Medtronic provided the glucose-monitoring equipment. Dr. Smart reported having no relevant financial disclosures.
At the American Diabetes Association scientific sessions, two studies were presented in adolescents with type 1 diabetes that show that postmeal glucose excursions were influenced by more than carbohydrates.
In adolescents with type 1 diabetes, higher fat and protein intake contributed to the rise in postmeal glucose levels using a continual glucose monitoring system, with less hypoglycemia after a higher-protein meal. Suggestions included using a dual wave bolus in pump patients before they eat a high fat meal or developing a new way besides carbohydrate counting to guesstimate insulin bolus requirements.
Similar in concept to the glycemic index, the food insulin demand (FID) is an algorithm for ranking foods based on their insulin demand and portion size relative to a reference food, and it takes into consideration protein intake.
It's likely that calculation of premeal insulin needs will consider a variety of factors to decrease the glucose excursions throughout the day: total food composition (carbohydrate, fat, and protein content) of anticipated meal, portion size, timing of when to bolus, adjustments for the premeal level of glucose (currently called the "correction factor"), anticipated exercise and stress post meal.
In other words, we have a long way to go to accurately predict glucose excursions and anticipate insulin requirements.
Dr. Jay Cohen is medical director of the Endocrine Clinic, Memphis, Tenn. He said that he had no financial disclosures relevant to this article.
At the American Diabetes Association scientific sessions, two studies were presented in adolescents with type 1 diabetes that show that postmeal glucose excursions were influenced by more than carbohydrates.
In adolescents with type 1 diabetes, higher fat and protein intake contributed to the rise in postmeal glucose levels using a continual glucose monitoring system, with less hypoglycemia after a higher-protein meal. Suggestions included using a dual wave bolus in pump patients before they eat a high fat meal or developing a new way besides carbohydrate counting to guesstimate insulin bolus requirements.
Similar in concept to the glycemic index, the food insulin demand (FID) is an algorithm for ranking foods based on their insulin demand and portion size relative to a reference food, and it takes into consideration protein intake.
It's likely that calculation of premeal insulin needs will consider a variety of factors to decrease the glucose excursions throughout the day: total food composition (carbohydrate, fat, and protein content) of anticipated meal, portion size, timing of when to bolus, adjustments for the premeal level of glucose (currently called the "correction factor"), anticipated exercise and stress post meal.
In other words, we have a long way to go to accurately predict glucose excursions and anticipate insulin requirements.
Dr. Jay Cohen is medical director of the Endocrine Clinic, Memphis, Tenn. He said that he had no financial disclosures relevant to this article.
At the American Diabetes Association scientific sessions, two studies were presented in adolescents with type 1 diabetes that show that postmeal glucose excursions were influenced by more than carbohydrates.
In adolescents with type 1 diabetes, higher fat and protein intake contributed to the rise in postmeal glucose levels using a continual glucose monitoring system, with less hypoglycemia after a higher-protein meal. Suggestions included using a dual wave bolus in pump patients before they eat a high fat meal or developing a new way besides carbohydrate counting to guesstimate insulin bolus requirements.
Similar in concept to the glycemic index, the food insulin demand (FID) is an algorithm for ranking foods based on their insulin demand and portion size relative to a reference food, and it takes into consideration protein intake.
It's likely that calculation of premeal insulin needs will consider a variety of factors to decrease the glucose excursions throughout the day: total food composition (carbohydrate, fat, and protein content) of anticipated meal, portion size, timing of when to bolus, adjustments for the premeal level of glucose (currently called the "correction factor"), anticipated exercise and stress post meal.
In other words, we have a long way to go to accurately predict glucose excursions and anticipate insulin requirements.
Dr. Jay Cohen is medical director of the Endocrine Clinic, Memphis, Tenn. He said that he had no financial disclosures relevant to this article.
CHICAGO – Meals high in fat or protein cause hyperglycemia among children using intensive insulin therapy for type 1 diabetes, a study has shown.
Further, protein and fat have an additive effect on postprandial glycemic rise, based on results of the randomized study involving 33 children.
"Protein and fat should be considered in prandial insulin dose and distribution," Carmel Smart, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
In intensive insulin therapy, the mealtime insulin dose is calculated based exclusively on counting carbohydrates. A growing body of evidence, however, suggests that macronutrients other than carbohydrates influence postprandial glucose levels and insulin requirements, said Dr. Smart, a specialist diabetes dietitian at the John Hunter Children’s Hospital, Newcastle, Australia.
She highlighted a recent study showing that high-fat meals with identical carbohydrate and protein content, required more insulin than low-fat meals and, despite the additional insulin, caused more hyperglycemia in type 1 diabetics (Diabetes Care 2013;36:810-6). An early study reported that meals with added protein significantly increased late glucose responses and insulin requirements, compared with standard or fat-added meals (Am. J. Clin. Nutr. 1993;58:555-60).
For the current experiment, 33 children (aged 8-17) ate four test pancake breakfasts on different days with identical carbohydrate content (30 g), but varying protein and fat: low fat (4 g)/low protein (5 g), high fat (35 g)/low protein, low fat/high protein (40 g), and high fat/high protein. A protein supplement and double cream (50% fat) were used to vary the protein and fat content.
The meals were given after the same individually standardized insulin dose, and no activity or snacks were allowed for 5 hours after the meal. Glucose excursions or fluctuations were measured at 30-minute increments for 5 hours using continuous glucose monitoring.
At baseline, the 17 girls and 16 boys had a mean A1c level of 7.2% and a mean duration of diabetes of 4.9 years; 27 were on continuous subcutaneous insulin infusion, and 6 were on a multiple daily insulin regimen. Their average age was 12.2 years.
Compared with the low-protein/low-fat meal, postprandial glucose excursions were significantly greater at 210-300 minutes after the high-fat/low-protein meal (1.78 mmol/L vs. –0.50 mmol/L; P = .01), Dr. Smart said.
Postprandial glucose excursions also were significantly greater at 180 minutes after the high-protein/low-fat meal (2.40 mmol/L vs. 0.54 mmol/L; P = .02).
The high-fat/high-protein meal resulted in significantly higher and sustained glucose excursions from 180 to 300 minutes compared with all the other meals (P less than .04).
The effect of fat and protein was additive at all time points after 150 minutes, she said. Compared with the low-fat/low-protein meal, the average glucose excursion was 4.2 mmol/L greater at 180 minutes after the high-fat/high-protein meal and 5.4 mmol/L greater at 5 hours.
Hypoglycemia, defined by a blood glucose level of less than 3.6 mmol/L, occurred in 29 children in the 5-hour postprandial period, and was significantly different between meal types (P = .003), Dr. Smart said.
There was a significant reduction in hypoglycemia after high-protein meals (odds ratio, 0.16; P less than .0001), but no reduction after high-fat meals (OR, 0.50; P = .08).
While meals high in protein and fat cause hyperglycemia, "our results suggest that protein may have a protective effect on hypoglycemia," she said.
During a discussion of the study, an audience member thanked the investigators for conducting the study "because this is what I’ve been seeing in patients eating high-fat diets, and I haven’t known exactly what to do about their insulin when they aren’t on the pump."
Dr. Smart agreed that they’ve been seeing this phenomenon for a long time, and said clinicians can now have the confidence to tell patients that it’s not inaccurate carbohydrate counting causing the glucose fluctuations, as previously believed. Currently, the investigators are advising patients on pump therapy to use the dual-wave bolus, although novel algorithms to determine prandial glucose dosing are being refined for high-fat/high-protein diets, she added.
A study led by Kirstine Bell, Ph.D., detailing one of those algorithms, called the food insulin index, was presented during the same session, by Dr. Stephen Colagiuri.
The study was supported by grants from Australian Pediatric Endocrine Care and Hunter Children’s Research Foundation. Medtronic provided the glucose-monitoring equipment. Dr. Smart reported having no relevant financial disclosures.
CHICAGO – Meals high in fat or protein cause hyperglycemia among children using intensive insulin therapy for type 1 diabetes, a study has shown.
Further, protein and fat have an additive effect on postprandial glycemic rise, based on results of the randomized study involving 33 children.
"Protein and fat should be considered in prandial insulin dose and distribution," Carmel Smart, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
In intensive insulin therapy, the mealtime insulin dose is calculated based exclusively on counting carbohydrates. A growing body of evidence, however, suggests that macronutrients other than carbohydrates influence postprandial glucose levels and insulin requirements, said Dr. Smart, a specialist diabetes dietitian at the John Hunter Children’s Hospital, Newcastle, Australia.
She highlighted a recent study showing that high-fat meals with identical carbohydrate and protein content, required more insulin than low-fat meals and, despite the additional insulin, caused more hyperglycemia in type 1 diabetics (Diabetes Care 2013;36:810-6). An early study reported that meals with added protein significantly increased late glucose responses and insulin requirements, compared with standard or fat-added meals (Am. J. Clin. Nutr. 1993;58:555-60).
For the current experiment, 33 children (aged 8-17) ate four test pancake breakfasts on different days with identical carbohydrate content (30 g), but varying protein and fat: low fat (4 g)/low protein (5 g), high fat (35 g)/low protein, low fat/high protein (40 g), and high fat/high protein. A protein supplement and double cream (50% fat) were used to vary the protein and fat content.
The meals were given after the same individually standardized insulin dose, and no activity or snacks were allowed for 5 hours after the meal. Glucose excursions or fluctuations were measured at 30-minute increments for 5 hours using continuous glucose monitoring.
At baseline, the 17 girls and 16 boys had a mean A1c level of 7.2% and a mean duration of diabetes of 4.9 years; 27 were on continuous subcutaneous insulin infusion, and 6 were on a multiple daily insulin regimen. Their average age was 12.2 years.
Compared with the low-protein/low-fat meal, postprandial glucose excursions were significantly greater at 210-300 minutes after the high-fat/low-protein meal (1.78 mmol/L vs. –0.50 mmol/L; P = .01), Dr. Smart said.
Postprandial glucose excursions also were significantly greater at 180 minutes after the high-protein/low-fat meal (2.40 mmol/L vs. 0.54 mmol/L; P = .02).
The high-fat/high-protein meal resulted in significantly higher and sustained glucose excursions from 180 to 300 minutes compared with all the other meals (P less than .04).
The effect of fat and protein was additive at all time points after 150 minutes, she said. Compared with the low-fat/low-protein meal, the average glucose excursion was 4.2 mmol/L greater at 180 minutes after the high-fat/high-protein meal and 5.4 mmol/L greater at 5 hours.
Hypoglycemia, defined by a blood glucose level of less than 3.6 mmol/L, occurred in 29 children in the 5-hour postprandial period, and was significantly different between meal types (P = .003), Dr. Smart said.
There was a significant reduction in hypoglycemia after high-protein meals (odds ratio, 0.16; P less than .0001), but no reduction after high-fat meals (OR, 0.50; P = .08).
While meals high in protein and fat cause hyperglycemia, "our results suggest that protein may have a protective effect on hypoglycemia," she said.
During a discussion of the study, an audience member thanked the investigators for conducting the study "because this is what I’ve been seeing in patients eating high-fat diets, and I haven’t known exactly what to do about their insulin when they aren’t on the pump."
Dr. Smart agreed that they’ve been seeing this phenomenon for a long time, and said clinicians can now have the confidence to tell patients that it’s not inaccurate carbohydrate counting causing the glucose fluctuations, as previously believed. Currently, the investigators are advising patients on pump therapy to use the dual-wave bolus, although novel algorithms to determine prandial glucose dosing are being refined for high-fat/high-protein diets, she added.
A study led by Kirstine Bell, Ph.D., detailing one of those algorithms, called the food insulin index, was presented during the same session, by Dr. Stephen Colagiuri.
The study was supported by grants from Australian Pediatric Endocrine Care and Hunter Children’s Research Foundation. Medtronic provided the glucose-monitoring equipment. Dr. Smart reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: A high-fat/high-protein meal resulted in significantly higher glucose excursions from 180 to 300 minutes, compared with all the other meals (P less than .04).
Data source: A randomized study in 33 children using intensive insulin therapy for type 1 diabetes.
Disclosures: The study was supported by grants from Australian Pediatric Endocrine Care and Hunter Children’s Research Foundation. Medtronic provided the glucose-monitoring equipment. Dr. Smart reported having no relevant financial disclosures.
Pilot study: Vitamin D promising for diabetic pain
CHICAGO – High-dose vitamin D2 supplementation shows promise for the treatment of chronic pain among women with type 2 diabetes and comorbid depression, according to a single-arm pilot study known as the Sunshine Study.
The vitamin D2 supplementation at 50,000 IU/week for 6 months also was associated with significant improvement in major depressive disorder in this uncontrolled study, Sue M. Penckofer, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
This was a small, hypothesis-generating study of a relatively low-cost and safe potential therapy for a common clinical condition. The encouraging results have led to a $1.5 million grant from the National Institutes of Health for a considerably larger 4-year randomized controlled trial due to begin later this year, said Dr. Penckofer, professor of nursing at Loyola University in Maywood, Ill.
The pilot study included 46 women with type 2 diabetes, major depressive disorder, and vitamin D insufficiency as evidenced by their mean baseline serum level of 18 ng/mL. They averaged 54.6 years of age, with a 7.8-year history of diabetes and a mean hemoglobin A1c of 6.8%. Twenty-eight of the women had neuropathic pain involving their legs and 34 had sensory pain, with numbness or tingling in their feet and/or legs.
After 6 months of weekly vitamin D supplementation, the subjects’ mean vitamin D blood level had climbed to 38 ng/mL. Their median score on the Center for Epidemiologic Studies Depression Scale improved from 26.8 at baseline to 12.2 and their depression rating on the Patient Health Questionnaire (PHQ-9) improved from 11.5 to 5.2, although their antidepressant medication regimens were not required to be held constant.
Scores on the neuropathic pain subscale of the Diabetes Symptom Checklist improved from a baseline of 3.2 to 1.8 at 3 months and 2.1 at 6 months. Scores on the sensory pain subscale improved from 7.3 at baseline to 5.0 at 3 months and 5.9 at 6 months. These favorable trends didn’t attain statistical significance in such a small study population, but patients in the top half of the group in terms of neuropathic or sensory pain did show statistically significant improvement over time. Those in the high baseline neuropathic pain group went from an average score of 5.2 at enrollment to 2.5 at 3 months and 2.8 at 6 months. Similarly, the high sensory pain subgroup improved from a baseline score of 10.6 to 6.8 at 3 months and 6.9 at 6 months, according to Dr. Penckofer.
The Sunshine Study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Penckofer reported having no conflicts of interest.
CHICAGO – High-dose vitamin D2 supplementation shows promise for the treatment of chronic pain among women with type 2 diabetes and comorbid depression, according to a single-arm pilot study known as the Sunshine Study.
The vitamin D2 supplementation at 50,000 IU/week for 6 months also was associated with significant improvement in major depressive disorder in this uncontrolled study, Sue M. Penckofer, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
This was a small, hypothesis-generating study of a relatively low-cost and safe potential therapy for a common clinical condition. The encouraging results have led to a $1.5 million grant from the National Institutes of Health for a considerably larger 4-year randomized controlled trial due to begin later this year, said Dr. Penckofer, professor of nursing at Loyola University in Maywood, Ill.
The pilot study included 46 women with type 2 diabetes, major depressive disorder, and vitamin D insufficiency as evidenced by their mean baseline serum level of 18 ng/mL. They averaged 54.6 years of age, with a 7.8-year history of diabetes and a mean hemoglobin A1c of 6.8%. Twenty-eight of the women had neuropathic pain involving their legs and 34 had sensory pain, with numbness or tingling in their feet and/or legs.
After 6 months of weekly vitamin D supplementation, the subjects’ mean vitamin D blood level had climbed to 38 ng/mL. Their median score on the Center for Epidemiologic Studies Depression Scale improved from 26.8 at baseline to 12.2 and their depression rating on the Patient Health Questionnaire (PHQ-9) improved from 11.5 to 5.2, although their antidepressant medication regimens were not required to be held constant.
Scores on the neuropathic pain subscale of the Diabetes Symptom Checklist improved from a baseline of 3.2 to 1.8 at 3 months and 2.1 at 6 months. Scores on the sensory pain subscale improved from 7.3 at baseline to 5.0 at 3 months and 5.9 at 6 months. These favorable trends didn’t attain statistical significance in such a small study population, but patients in the top half of the group in terms of neuropathic or sensory pain did show statistically significant improvement over time. Those in the high baseline neuropathic pain group went from an average score of 5.2 at enrollment to 2.5 at 3 months and 2.8 at 6 months. Similarly, the high sensory pain subgroup improved from a baseline score of 10.6 to 6.8 at 3 months and 6.9 at 6 months, according to Dr. Penckofer.
The Sunshine Study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Penckofer reported having no conflicts of interest.
CHICAGO – High-dose vitamin D2 supplementation shows promise for the treatment of chronic pain among women with type 2 diabetes and comorbid depression, according to a single-arm pilot study known as the Sunshine Study.
The vitamin D2 supplementation at 50,000 IU/week for 6 months also was associated with significant improvement in major depressive disorder in this uncontrolled study, Sue M. Penckofer, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
This was a small, hypothesis-generating study of a relatively low-cost and safe potential therapy for a common clinical condition. The encouraging results have led to a $1.5 million grant from the National Institutes of Health for a considerably larger 4-year randomized controlled trial due to begin later this year, said Dr. Penckofer, professor of nursing at Loyola University in Maywood, Ill.
The pilot study included 46 women with type 2 diabetes, major depressive disorder, and vitamin D insufficiency as evidenced by their mean baseline serum level of 18 ng/mL. They averaged 54.6 years of age, with a 7.8-year history of diabetes and a mean hemoglobin A1c of 6.8%. Twenty-eight of the women had neuropathic pain involving their legs and 34 had sensory pain, with numbness or tingling in their feet and/or legs.
After 6 months of weekly vitamin D supplementation, the subjects’ mean vitamin D blood level had climbed to 38 ng/mL. Their median score on the Center for Epidemiologic Studies Depression Scale improved from 26.8 at baseline to 12.2 and their depression rating on the Patient Health Questionnaire (PHQ-9) improved from 11.5 to 5.2, although their antidepressant medication regimens were not required to be held constant.
Scores on the neuropathic pain subscale of the Diabetes Symptom Checklist improved from a baseline of 3.2 to 1.8 at 3 months and 2.1 at 6 months. Scores on the sensory pain subscale improved from 7.3 at baseline to 5.0 at 3 months and 5.9 at 6 months. These favorable trends didn’t attain statistical significance in such a small study population, but patients in the top half of the group in terms of neuropathic or sensory pain did show statistically significant improvement over time. Those in the high baseline neuropathic pain group went from an average score of 5.2 at enrollment to 2.5 at 3 months and 2.8 at 6 months. Similarly, the high sensory pain subgroup improved from a baseline score of 10.6 to 6.8 at 3 months and 6.9 at 6 months, according to Dr. Penckofer.
The Sunshine Study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Penckofer reported having no conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Type 2 diabetes patients with comorbid depression and baseline elevated neuropathic and/or sensory pain symptoms showed significant improvement in pain scores after 6 months of high-dose vitamin D2 supplementation.
Data source: The Sunshine Study was a prospective, uncontrolled study in which 46 women with type 2 diabetes, comorbid major depression and chronic pain, and vitamin D insufficiency received 50,000 IU/week of vitamin D2 for 6 months.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Penckofer reported having no conflicts of interest.
Children of diabetic mothers at greater risk of overweight as young adults
CHICAGO – At age 17, the children of mothers with diabetes during pregnancy are more likely to be overweight, according to an Israeli longitudinal study.
This was equally the case regardless of whether the mother had gestational or pregestational diabetes, Dr. Zvi Laron reported at the annual scientific sessions of the American Diabetes Association.
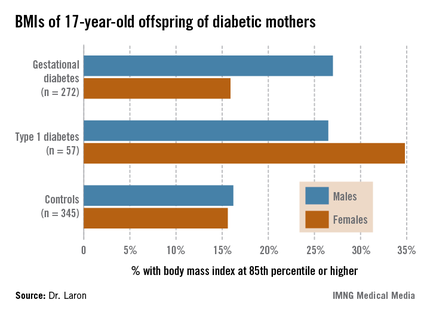
The Israeli data expand upon the earlier findings of EPOCH (Exploring Perinatal Outcomes among Children), a retrospective cohort study conducted by investigators at the University of Colorado, Denver. The Colorado investigators followed 95 children exposed to diabetes in utero – 87 whose mothers had gestational diabetes and 8 with type 1 diabetes – and 409 controls out to age 13 years, and determined that the children of diabetic mothers were more obese. Of note, the investigators found no significant differences between the children of diabetic mothers and controls in growth trajectories during infancy and early childhood, but a higher body mass index growth velocity among the offspring of diabetic mothers at ages 10-13 years (J. Pediatr. 2011;158:941-6).
The Israeli study tracked a much larger group of children of diabetic mothers later into adolescence, taking advantage of the fact that most Israeli youth have to report for compulsory military service at age 17.
Dr. Laron of Tel Aviv University and his coworkers identified 447 singleton term neonates of mothers with gestational diabetes and 97 whose mothers had type 1 diabetes born during 1987-1993 at the Helen Schneider Hospital for Women in Petach Tikva, Israel. The control group was composed of an equal number of singleton term babies born to nondiabetic mothers at the hospital during the same years.
Consistent with other reports, the average birth weight and length of the babies of diabetic mothers were significantly greater than in controls. For example, 24.3% of the baby boys and 18.4% of the girls of mothers with gestational diabetes were at or above the 90th percentile for birth weight for the Israeli population, compared with 6.8% of male and 6.0% of female controls.
Sixty-two percent of the original study population presented for military service at age 17; Arabs, as well as ultra-Orthodox Jewish men and women, are exempt from military service. The offspring exposed in utero to maternal diabetes didn’t differ from controls in terms of height, but the males with in utero exposure were significantly more likely to have a BMI at or above the 85th percentile nationally, and the females showed a similar albeit nonsignificant trend (see chart). Unlike in the Colorado study, however, the Israeli investigators didn’t have access to serial measurements that would have enabled them to determine the age at which the accelerated BMI growth trajectory occurred.
It is well established that adolescent overweight is associated with increased risk of subsequent obesity in adulthood. If the Colorado investigators are correct in their conclusion that exposure to diabetes in utero results in accelerated BMI growth occurring in late childhood, it would suggest that early childhood provides a window of opportunity for obesity prevention in these children. But a better understanding of the underlying mechanisms involved in the association between in utero exposure to diabetes and accelerated BMI growth trajectory occurring 10-13 years later may be needed before targeted preventive interventions can be developed and tested.
Dr. Laron reported having no conflicts of interest.
CHICAGO – At age 17, the children of mothers with diabetes during pregnancy are more likely to be overweight, according to an Israeli longitudinal study.
This was equally the case regardless of whether the mother had gestational or pregestational diabetes, Dr. Zvi Laron reported at the annual scientific sessions of the American Diabetes Association.

The Israeli data expand upon the earlier findings of EPOCH (Exploring Perinatal Outcomes among Children), a retrospective cohort study conducted by investigators at the University of Colorado, Denver. The Colorado investigators followed 95 children exposed to diabetes in utero – 87 whose mothers had gestational diabetes and 8 with type 1 diabetes – and 409 controls out to age 13 years, and determined that the children of diabetic mothers were more obese. Of note, the investigators found no significant differences between the children of diabetic mothers and controls in growth trajectories during infancy and early childhood, but a higher body mass index growth velocity among the offspring of diabetic mothers at ages 10-13 years (J. Pediatr. 2011;158:941-6).
The Israeli study tracked a much larger group of children of diabetic mothers later into adolescence, taking advantage of the fact that most Israeli youth have to report for compulsory military service at age 17.
Dr. Laron of Tel Aviv University and his coworkers identified 447 singleton term neonates of mothers with gestational diabetes and 97 whose mothers had type 1 diabetes born during 1987-1993 at the Helen Schneider Hospital for Women in Petach Tikva, Israel. The control group was composed of an equal number of singleton term babies born to nondiabetic mothers at the hospital during the same years.
Consistent with other reports, the average birth weight and length of the babies of diabetic mothers were significantly greater than in controls. For example, 24.3% of the baby boys and 18.4% of the girls of mothers with gestational diabetes were at or above the 90th percentile for birth weight for the Israeli population, compared with 6.8% of male and 6.0% of female controls.
Sixty-two percent of the original study population presented for military service at age 17; Arabs, as well as ultra-Orthodox Jewish men and women, are exempt from military service. The offspring exposed in utero to maternal diabetes didn’t differ from controls in terms of height, but the males with in utero exposure were significantly more likely to have a BMI at or above the 85th percentile nationally, and the females showed a similar albeit nonsignificant trend (see chart). Unlike in the Colorado study, however, the Israeli investigators didn’t have access to serial measurements that would have enabled them to determine the age at which the accelerated BMI growth trajectory occurred.
It is well established that adolescent overweight is associated with increased risk of subsequent obesity in adulthood. If the Colorado investigators are correct in their conclusion that exposure to diabetes in utero results in accelerated BMI growth occurring in late childhood, it would suggest that early childhood provides a window of opportunity for obesity prevention in these children. But a better understanding of the underlying mechanisms involved in the association between in utero exposure to diabetes and accelerated BMI growth trajectory occurring 10-13 years later may be needed before targeted preventive interventions can be developed and tested.
Dr. Laron reported having no conflicts of interest.
CHICAGO – At age 17, the children of mothers with diabetes during pregnancy are more likely to be overweight, according to an Israeli longitudinal study.
This was equally the case regardless of whether the mother had gestational or pregestational diabetes, Dr. Zvi Laron reported at the annual scientific sessions of the American Diabetes Association.

The Israeli data expand upon the earlier findings of EPOCH (Exploring Perinatal Outcomes among Children), a retrospective cohort study conducted by investigators at the University of Colorado, Denver. The Colorado investigators followed 95 children exposed to diabetes in utero – 87 whose mothers had gestational diabetes and 8 with type 1 diabetes – and 409 controls out to age 13 years, and determined that the children of diabetic mothers were more obese. Of note, the investigators found no significant differences between the children of diabetic mothers and controls in growth trajectories during infancy and early childhood, but a higher body mass index growth velocity among the offspring of diabetic mothers at ages 10-13 years (J. Pediatr. 2011;158:941-6).
The Israeli study tracked a much larger group of children of diabetic mothers later into adolescence, taking advantage of the fact that most Israeli youth have to report for compulsory military service at age 17.
Dr. Laron of Tel Aviv University and his coworkers identified 447 singleton term neonates of mothers with gestational diabetes and 97 whose mothers had type 1 diabetes born during 1987-1993 at the Helen Schneider Hospital for Women in Petach Tikva, Israel. The control group was composed of an equal number of singleton term babies born to nondiabetic mothers at the hospital during the same years.
Consistent with other reports, the average birth weight and length of the babies of diabetic mothers were significantly greater than in controls. For example, 24.3% of the baby boys and 18.4% of the girls of mothers with gestational diabetes were at or above the 90th percentile for birth weight for the Israeli population, compared with 6.8% of male and 6.0% of female controls.
Sixty-two percent of the original study population presented for military service at age 17; Arabs, as well as ultra-Orthodox Jewish men and women, are exempt from military service. The offspring exposed in utero to maternal diabetes didn’t differ from controls in terms of height, but the males with in utero exposure were significantly more likely to have a BMI at or above the 85th percentile nationally, and the females showed a similar albeit nonsignificant trend (see chart). Unlike in the Colorado study, however, the Israeli investigators didn’t have access to serial measurements that would have enabled them to determine the age at which the accelerated BMI growth trajectory occurred.
It is well established that adolescent overweight is associated with increased risk of subsequent obesity in adulthood. If the Colorado investigators are correct in their conclusion that exposure to diabetes in utero results in accelerated BMI growth occurring in late childhood, it would suggest that early childhood provides a window of opportunity for obesity prevention in these children. But a better understanding of the underlying mechanisms involved in the association between in utero exposure to diabetes and accelerated BMI growth trajectory occurring 10-13 years later may be needed before targeted preventive interventions can be developed and tested.
Dr. Laron reported having no conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Of 17-year-old males born to diabetic mothers, 27% had a BMI at or above the 85th percentile nationally, compared with 16% of controls.
Data source: A retrospective study of 329 Israeli 17-year-olds born to diabetic mothers and 345 controls.
Disclosures: Dr. Laron reported having no conflicts of interest.
Two meals per day better than six in T2DM
CHICAGO – Overweight patients with type 2 diabetes who ate only breakfast and lunch lost more weight than did those eating six meals a day in a randomized, crossover study.
They also had greater decreases in fasting plasma glucose, C-peptide, glucagon, and hepatic fat content, and bigger improvements in insulin sensitivity.
"Our data suggest that consuming a hearty breakfast and lunch may be more beneficial for patients with type 2 diabetes than eating more, smaller meals during the day," Dr. Hana Kahleová, said at the annual scientific sessions of the American Diabetes Association.
When asked whether eating a big lunch and dinner would produce the same benefits given the difficulty clinicians would face convincing patients to give up their evening meal, Dr. Kahleová agreed it would be a hard sell, but said the breakfast/lunch schedule is more beneficial. She highlighted research during the presentation showing fat deposition is greater following dinner (J. Clin. Endocrinol. Metab. 2009;94:1781-8), and that diabetes-free people of Mediterranean heritage who ate most of their daily intake early lost more weight and did so at a faster rate than did late eaters (Int. J. Obes. [Lond.] 2013;37:604-11).
"Our results support the ancient proverb: Eat breakfast like a king, lunch like a prince, and dinner like a pauper," said Dr. Kahleová of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Session moderator Dr. Anastassios Pittas, codirector of the Diabetes Center at Tufts Medical Center in Boston, said in an interview that few clinicians are in favor of six small meals a day because invariably patients will eat six moderate-size meals. Insulin dosing with such frequent meals is also more onerous.
"I agree with the finding that two is better than six, but that does not imply that, in general, the concept of fewer meals is better than more meals," he said. "It’s just that the comparison groups were so extreme that it’s logical to me what happened. I think the better, clinically applicable comparison would have been two vs. four or maybe five meals."
The six-meal diet was chosen as the comparator because it has been usually recommended that patients with type 2 diabetes eat five to six small meals during the day, Dr. Kahleová said in an interview. Although diabetic patients who eat small regular meals are reported to have better control of their blood glucose concentrations, the current results are strongly supported by animal studies showing the antidiabetic effects of caloric restriction and intermittent fasting diets. These effects include reduced blood glucose and insulin concentrations and improved glucose tolerance in rodents and are thought to be driven largely by increased insulin sensitivity.
Dr. Kahleová and her associates evenly randomized 54 patients with type 2 diabetes for more than 1 year to follow a six meals/day or two meals/day diet for 12 weeks. The diets were then switched for a subsequent 12 weeks. Both diets had the same caloric restriction (–500 kcal/day), carbohydrates (50%-55%), proteins (20%-25%), fats (25%-30%), cholesterol (less than 200 mg/day), and fiber (30g-40g/day).
For the two-meal diet, breakfast was eaten between hours 6-10 and lunch between hours 12-16. In the six-meal diet, breakfast was eaten during hours 6-8, snack hours 8-10, lunch hours 11-13, second snack hours 14-15, dinner hours 17-18 and second dinner hours 20-21.
To improve compliance, each regimen was started with a 4-day tutorial on how to compose and prepare their diet; group meetings with a dietician; individual counseling and meals provided for half of the patients. Patients were also asked not to alter their exercise habits during the study.
At baseline, 54% of patients were female, average diabetes duration 8.1 years, average body mass index 32.6 kg/m2, and average hemoglobin A1c 7.2%. Their average age was 59.4 years.
At 12 weeks, the mean change in BMI significantly favored the two-meal diet over the six-meal diet (–1.23 kg/m2 vs.–0.82 kg/m2; P less than .001), Dr. Kahleová said. Waist circumference shrunk significantly more as well (–5.14 cm vs. –1.37 cm; P less than .001).
Fasting plasma glucose decreased in response to both diets, but the decrease was significantly greater with two meals per day (–0.78 mmol/L vs. –0.47 mmol/L; P = .004), she said. C-peptide followed the same pattern (P = .04).
Hepatic fat content, as measured by proton magnetic resonance spectroscopy, decreased significantly more with two meals per day (–4.2% vs. –3.4%; P less than .001).
HbA1c decreased comparably in both diets, Dr. Kahleová said. Fasting plasma glucagon decreased with two daily meals (P less than .001), while it increased with six meals (P = .04).
Both parameters of beta-cell function – insulin secretion and glucose sensitivity – increased comparably with the two diets, Dr. Kahleová said. Changes in glucose sensitivity (P = .02) and oral glucose insulin sensitivity (P less than .001) correlated negatively with the change in hepatic fat content. The correlations were no longer significant, however, after adjustment for changes in BMI.
Dr. Kahleová said the results were as expected, and she declined to speculate as to whether they would be the same if the cohort had been heavier than allowed with the BMI inclusion criteria of 27-40 kg/m2.
The investigators are currently trying to find a link between postprandial oxidative stress and gastrointestinal peptides in patients with type 2 diabetes.
The study was supported by a grant from the Ministry of Health, Prague, and by the Grant Agency of Charles University, also in Prague. Dr. Kahleová reported having no financial disclosures.
CHICAGO – Overweight patients with type 2 diabetes who ate only breakfast and lunch lost more weight than did those eating six meals a day in a randomized, crossover study.
They also had greater decreases in fasting plasma glucose, C-peptide, glucagon, and hepatic fat content, and bigger improvements in insulin sensitivity.
"Our data suggest that consuming a hearty breakfast and lunch may be more beneficial for patients with type 2 diabetes than eating more, smaller meals during the day," Dr. Hana Kahleová, said at the annual scientific sessions of the American Diabetes Association.
When asked whether eating a big lunch and dinner would produce the same benefits given the difficulty clinicians would face convincing patients to give up their evening meal, Dr. Kahleová agreed it would be a hard sell, but said the breakfast/lunch schedule is more beneficial. She highlighted research during the presentation showing fat deposition is greater following dinner (J. Clin. Endocrinol. Metab. 2009;94:1781-8), and that diabetes-free people of Mediterranean heritage who ate most of their daily intake early lost more weight and did so at a faster rate than did late eaters (Int. J. Obes. [Lond.] 2013;37:604-11).
"Our results support the ancient proverb: Eat breakfast like a king, lunch like a prince, and dinner like a pauper," said Dr. Kahleová of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Session moderator Dr. Anastassios Pittas, codirector of the Diabetes Center at Tufts Medical Center in Boston, said in an interview that few clinicians are in favor of six small meals a day because invariably patients will eat six moderate-size meals. Insulin dosing with such frequent meals is also more onerous.
"I agree with the finding that two is better than six, but that does not imply that, in general, the concept of fewer meals is better than more meals," he said. "It’s just that the comparison groups were so extreme that it’s logical to me what happened. I think the better, clinically applicable comparison would have been two vs. four or maybe five meals."
The six-meal diet was chosen as the comparator because it has been usually recommended that patients with type 2 diabetes eat five to six small meals during the day, Dr. Kahleová said in an interview. Although diabetic patients who eat small regular meals are reported to have better control of their blood glucose concentrations, the current results are strongly supported by animal studies showing the antidiabetic effects of caloric restriction and intermittent fasting diets. These effects include reduced blood glucose and insulin concentrations and improved glucose tolerance in rodents and are thought to be driven largely by increased insulin sensitivity.
Dr. Kahleová and her associates evenly randomized 54 patients with type 2 diabetes for more than 1 year to follow a six meals/day or two meals/day diet for 12 weeks. The diets were then switched for a subsequent 12 weeks. Both diets had the same caloric restriction (–500 kcal/day), carbohydrates (50%-55%), proteins (20%-25%), fats (25%-30%), cholesterol (less than 200 mg/day), and fiber (30g-40g/day).
For the two-meal diet, breakfast was eaten between hours 6-10 and lunch between hours 12-16. In the six-meal diet, breakfast was eaten during hours 6-8, snack hours 8-10, lunch hours 11-13, second snack hours 14-15, dinner hours 17-18 and second dinner hours 20-21.
To improve compliance, each regimen was started with a 4-day tutorial on how to compose and prepare their diet; group meetings with a dietician; individual counseling and meals provided for half of the patients. Patients were also asked not to alter their exercise habits during the study.
At baseline, 54% of patients were female, average diabetes duration 8.1 years, average body mass index 32.6 kg/m2, and average hemoglobin A1c 7.2%. Their average age was 59.4 years.
At 12 weeks, the mean change in BMI significantly favored the two-meal diet over the six-meal diet (–1.23 kg/m2 vs.–0.82 kg/m2; P less than .001), Dr. Kahleová said. Waist circumference shrunk significantly more as well (–5.14 cm vs. –1.37 cm; P less than .001).
Fasting plasma glucose decreased in response to both diets, but the decrease was significantly greater with two meals per day (–0.78 mmol/L vs. –0.47 mmol/L; P = .004), she said. C-peptide followed the same pattern (P = .04).
Hepatic fat content, as measured by proton magnetic resonance spectroscopy, decreased significantly more with two meals per day (–4.2% vs. –3.4%; P less than .001).
HbA1c decreased comparably in both diets, Dr. Kahleová said. Fasting plasma glucagon decreased with two daily meals (P less than .001), while it increased with six meals (P = .04).
Both parameters of beta-cell function – insulin secretion and glucose sensitivity – increased comparably with the two diets, Dr. Kahleová said. Changes in glucose sensitivity (P = .02) and oral glucose insulin sensitivity (P less than .001) correlated negatively with the change in hepatic fat content. The correlations were no longer significant, however, after adjustment for changes in BMI.
Dr. Kahleová said the results were as expected, and she declined to speculate as to whether they would be the same if the cohort had been heavier than allowed with the BMI inclusion criteria of 27-40 kg/m2.
The investigators are currently trying to find a link between postprandial oxidative stress and gastrointestinal peptides in patients with type 2 diabetes.
The study was supported by a grant from the Ministry of Health, Prague, and by the Grant Agency of Charles University, also in Prague. Dr. Kahleová reported having no financial disclosures.
CHICAGO – Overweight patients with type 2 diabetes who ate only breakfast and lunch lost more weight than did those eating six meals a day in a randomized, crossover study.
They also had greater decreases in fasting plasma glucose, C-peptide, glucagon, and hepatic fat content, and bigger improvements in insulin sensitivity.
"Our data suggest that consuming a hearty breakfast and lunch may be more beneficial for patients with type 2 diabetes than eating more, smaller meals during the day," Dr. Hana Kahleová, said at the annual scientific sessions of the American Diabetes Association.
When asked whether eating a big lunch and dinner would produce the same benefits given the difficulty clinicians would face convincing patients to give up their evening meal, Dr. Kahleová agreed it would be a hard sell, but said the breakfast/lunch schedule is more beneficial. She highlighted research during the presentation showing fat deposition is greater following dinner (J. Clin. Endocrinol. Metab. 2009;94:1781-8), and that diabetes-free people of Mediterranean heritage who ate most of their daily intake early lost more weight and did so at a faster rate than did late eaters (Int. J. Obes. [Lond.] 2013;37:604-11).
"Our results support the ancient proverb: Eat breakfast like a king, lunch like a prince, and dinner like a pauper," said Dr. Kahleová of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Session moderator Dr. Anastassios Pittas, codirector of the Diabetes Center at Tufts Medical Center in Boston, said in an interview that few clinicians are in favor of six small meals a day because invariably patients will eat six moderate-size meals. Insulin dosing with such frequent meals is also more onerous.
"I agree with the finding that two is better than six, but that does not imply that, in general, the concept of fewer meals is better than more meals," he said. "It’s just that the comparison groups were so extreme that it’s logical to me what happened. I think the better, clinically applicable comparison would have been two vs. four or maybe five meals."
The six-meal diet was chosen as the comparator because it has been usually recommended that patients with type 2 diabetes eat five to six small meals during the day, Dr. Kahleová said in an interview. Although diabetic patients who eat small regular meals are reported to have better control of their blood glucose concentrations, the current results are strongly supported by animal studies showing the antidiabetic effects of caloric restriction and intermittent fasting diets. These effects include reduced blood glucose and insulin concentrations and improved glucose tolerance in rodents and are thought to be driven largely by increased insulin sensitivity.
Dr. Kahleová and her associates evenly randomized 54 patients with type 2 diabetes for more than 1 year to follow a six meals/day or two meals/day diet for 12 weeks. The diets were then switched for a subsequent 12 weeks. Both diets had the same caloric restriction (–500 kcal/day), carbohydrates (50%-55%), proteins (20%-25%), fats (25%-30%), cholesterol (less than 200 mg/day), and fiber (30g-40g/day).
For the two-meal diet, breakfast was eaten between hours 6-10 and lunch between hours 12-16. In the six-meal diet, breakfast was eaten during hours 6-8, snack hours 8-10, lunch hours 11-13, second snack hours 14-15, dinner hours 17-18 and second dinner hours 20-21.
To improve compliance, each regimen was started with a 4-day tutorial on how to compose and prepare their diet; group meetings with a dietician; individual counseling and meals provided for half of the patients. Patients were also asked not to alter their exercise habits during the study.
At baseline, 54% of patients were female, average diabetes duration 8.1 years, average body mass index 32.6 kg/m2, and average hemoglobin A1c 7.2%. Their average age was 59.4 years.
At 12 weeks, the mean change in BMI significantly favored the two-meal diet over the six-meal diet (–1.23 kg/m2 vs.–0.82 kg/m2; P less than .001), Dr. Kahleová said. Waist circumference shrunk significantly more as well (–5.14 cm vs. –1.37 cm; P less than .001).
Fasting plasma glucose decreased in response to both diets, but the decrease was significantly greater with two meals per day (–0.78 mmol/L vs. –0.47 mmol/L; P = .004), she said. C-peptide followed the same pattern (P = .04).
Hepatic fat content, as measured by proton magnetic resonance spectroscopy, decreased significantly more with two meals per day (–4.2% vs. –3.4%; P less than .001).
HbA1c decreased comparably in both diets, Dr. Kahleová said. Fasting plasma glucagon decreased with two daily meals (P less than .001), while it increased with six meals (P = .04).
Both parameters of beta-cell function – insulin secretion and glucose sensitivity – increased comparably with the two diets, Dr. Kahleová said. Changes in glucose sensitivity (P = .02) and oral glucose insulin sensitivity (P less than .001) correlated negatively with the change in hepatic fat content. The correlations were no longer significant, however, after adjustment for changes in BMI.
Dr. Kahleová said the results were as expected, and she declined to speculate as to whether they would be the same if the cohort had been heavier than allowed with the BMI inclusion criteria of 27-40 kg/m2.
The investigators are currently trying to find a link between postprandial oxidative stress and gastrointestinal peptides in patients with type 2 diabetes.
The study was supported by a grant from the Ministry of Health, Prague, and by the Grant Agency of Charles University, also in Prague. Dr. Kahleová reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: The mean change in BMI at 12 weeks with two meals was –1.23 kg/m2 vs. –0.82 kg/m2 with six meals (P less than .001).
Data source: Randomized, cross-over study in 54 patients with type 2 diabetes.
Disclosures: The study was supported by a grant from the Ministry of Health, Prague, and by the Grant Agency of Charles University, also in Prague. Dr. Kahleová reported having no financial disclosures.
Ketoacidosis rates remain troubling in juvenile type 1 diabetes
CHICAGO – The prevalence of diabetic ketoacidosis has fallen by about 10% annually over time in youth with type 2 diabetes, but the life-threatening condition continues to plague roughly one in three children with type 1 diabetes, according to Dr. Dana Dabelea.
"The frequency of diabetic ketoacidosis in youth with type 1 diabetes, while stable, remains high among U.S. children at one in three children, which indicates a persistent need for increased awareness and better parental education, but also better access to health care," Dr. Dana Dabelea said at the annual scientific sessions of the American Diabetes Association.
She reported on an analysis involving 5,618 youth, aged less than 20 years, with newly diagnosed diabetes in three time periods (2002-2003, 2004-2005, and 2008-2009) and enrolled in the SEARCH for Diabetes in Youth study, a national, multicenter study designed to learn more about diabetes among American children and young adults.
Diabetic ketoacidosis (DKA) was defined by ICD-9 code and/or bicarbonate levels less than 15 mmol/L and/or a venous pH less than 7.25. Based on provider assessment, 4,537 patients had type 1 diabetes and 1,081 had type 2.
U.S. trend data on DKA prevalence in type 1 diabetes are limited, while no data are available in youth with type 2 diabetes, said Dr. Dabelea, professor of epidemiology, Colorado School for Public Health, Aurora. In Europe, decreases in DKA prevalence at onset of type 1 diabetes have been reported in Sweden and Finland, but not in Germany and Austria.
In the current analysis, DKA prevalence among youth with type 1 diabetes remained high at 29.8%, 28.8%, and 28.2% between 2002 and 2003, 2004 and 2005, and 2008 and 2009 (P = .43), she said. The number of patients diagnosed was 434, 453, and 425, respectively.
For type 2 diabetes, DKA prevalence declined from 11.3% to 6.5%, and then 6.1% over the same time periods. The difference was statistically significant (P = .02), but the number of patients is very small at 37, 21, and 26, Dr. Dabelea reported.
"In youth with type 2 diabetes, DKA at onset is less common and seems to be decreasing over time, suggesting perhaps improved diagnosis in at-risk individuals," Dr. Dabelea said.
The number of patients was too small for stratified type 2 analyses. For type 1 diabetes, DKA prevalence did not vary significantly by age at onset, race, or gender, although children aged 0-4 years in addition to blacks and Hispanics had significantly higher rates in all three time periods, she said.
In multivariate analyses, higher DKA prevalence in type 1 diabetes was independently associated with younger age at onset (age 5-9, odds ratio 0.57; age 10-14, OR 0.75; age 15-19, OR 0.38; all P less than .0001 compared with age 0-4), minority race (OR 1.33; P = .002), and lack of private insurance (OR 1.33; P = .010). Gender, education, and income were not significantly associated with a higher DKA, Dr. Dabelea reported.
Independent correlates of DKA in type 2 diabetes were younger age at onset (age 15-19 vs. age 10-14: OR 0.48; P = .002), minority race (OR 2.45; P = .013), and male gender (OR 0.54; P = .008).
"With changes in health care access on the horizon through health care reform, we might begin to see future decreases in DKA prevalence over time, but we believe that focused efforts to help the minority and underserved populations will be needed to achieve this goal," Dr. Dabelea concluded.
SEARCH is funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Dabelea reported having no relevant financial disclosures.
CHICAGO – The prevalence of diabetic ketoacidosis has fallen by about 10% annually over time in youth with type 2 diabetes, but the life-threatening condition continues to plague roughly one in three children with type 1 diabetes, according to Dr. Dana Dabelea.
"The frequency of diabetic ketoacidosis in youth with type 1 diabetes, while stable, remains high among U.S. children at one in three children, which indicates a persistent need for increased awareness and better parental education, but also better access to health care," Dr. Dana Dabelea said at the annual scientific sessions of the American Diabetes Association.
She reported on an analysis involving 5,618 youth, aged less than 20 years, with newly diagnosed diabetes in three time periods (2002-2003, 2004-2005, and 2008-2009) and enrolled in the SEARCH for Diabetes in Youth study, a national, multicenter study designed to learn more about diabetes among American children and young adults.
Diabetic ketoacidosis (DKA) was defined by ICD-9 code and/or bicarbonate levels less than 15 mmol/L and/or a venous pH less than 7.25. Based on provider assessment, 4,537 patients had type 1 diabetes and 1,081 had type 2.
U.S. trend data on DKA prevalence in type 1 diabetes are limited, while no data are available in youth with type 2 diabetes, said Dr. Dabelea, professor of epidemiology, Colorado School for Public Health, Aurora. In Europe, decreases in DKA prevalence at onset of type 1 diabetes have been reported in Sweden and Finland, but not in Germany and Austria.
In the current analysis, DKA prevalence among youth with type 1 diabetes remained high at 29.8%, 28.8%, and 28.2% between 2002 and 2003, 2004 and 2005, and 2008 and 2009 (P = .43), she said. The number of patients diagnosed was 434, 453, and 425, respectively.
For type 2 diabetes, DKA prevalence declined from 11.3% to 6.5%, and then 6.1% over the same time periods. The difference was statistically significant (P = .02), but the number of patients is very small at 37, 21, and 26, Dr. Dabelea reported.
"In youth with type 2 diabetes, DKA at onset is less common and seems to be decreasing over time, suggesting perhaps improved diagnosis in at-risk individuals," Dr. Dabelea said.
The number of patients was too small for stratified type 2 analyses. For type 1 diabetes, DKA prevalence did not vary significantly by age at onset, race, or gender, although children aged 0-4 years in addition to blacks and Hispanics had significantly higher rates in all three time periods, she said.
In multivariate analyses, higher DKA prevalence in type 1 diabetes was independently associated with younger age at onset (age 5-9, odds ratio 0.57; age 10-14, OR 0.75; age 15-19, OR 0.38; all P less than .0001 compared with age 0-4), minority race (OR 1.33; P = .002), and lack of private insurance (OR 1.33; P = .010). Gender, education, and income were not significantly associated with a higher DKA, Dr. Dabelea reported.
Independent correlates of DKA in type 2 diabetes were younger age at onset (age 15-19 vs. age 10-14: OR 0.48; P = .002), minority race (OR 2.45; P = .013), and male gender (OR 0.54; P = .008).
"With changes in health care access on the horizon through health care reform, we might begin to see future decreases in DKA prevalence over time, but we believe that focused efforts to help the minority and underserved populations will be needed to achieve this goal," Dr. Dabelea concluded.
SEARCH is funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Dabelea reported having no relevant financial disclosures.
CHICAGO – The prevalence of diabetic ketoacidosis has fallen by about 10% annually over time in youth with type 2 diabetes, but the life-threatening condition continues to plague roughly one in three children with type 1 diabetes, according to Dr. Dana Dabelea.
"The frequency of diabetic ketoacidosis in youth with type 1 diabetes, while stable, remains high among U.S. children at one in three children, which indicates a persistent need for increased awareness and better parental education, but also better access to health care," Dr. Dana Dabelea said at the annual scientific sessions of the American Diabetes Association.
She reported on an analysis involving 5,618 youth, aged less than 20 years, with newly diagnosed diabetes in three time periods (2002-2003, 2004-2005, and 2008-2009) and enrolled in the SEARCH for Diabetes in Youth study, a national, multicenter study designed to learn more about diabetes among American children and young adults.
Diabetic ketoacidosis (DKA) was defined by ICD-9 code and/or bicarbonate levels less than 15 mmol/L and/or a venous pH less than 7.25. Based on provider assessment, 4,537 patients had type 1 diabetes and 1,081 had type 2.
U.S. trend data on DKA prevalence in type 1 diabetes are limited, while no data are available in youth with type 2 diabetes, said Dr. Dabelea, professor of epidemiology, Colorado School for Public Health, Aurora. In Europe, decreases in DKA prevalence at onset of type 1 diabetes have been reported in Sweden and Finland, but not in Germany and Austria.
In the current analysis, DKA prevalence among youth with type 1 diabetes remained high at 29.8%, 28.8%, and 28.2% between 2002 and 2003, 2004 and 2005, and 2008 and 2009 (P = .43), she said. The number of patients diagnosed was 434, 453, and 425, respectively.
For type 2 diabetes, DKA prevalence declined from 11.3% to 6.5%, and then 6.1% over the same time periods. The difference was statistically significant (P = .02), but the number of patients is very small at 37, 21, and 26, Dr. Dabelea reported.
"In youth with type 2 diabetes, DKA at onset is less common and seems to be decreasing over time, suggesting perhaps improved diagnosis in at-risk individuals," Dr. Dabelea said.
The number of patients was too small for stratified type 2 analyses. For type 1 diabetes, DKA prevalence did not vary significantly by age at onset, race, or gender, although children aged 0-4 years in addition to blacks and Hispanics had significantly higher rates in all three time periods, she said.
In multivariate analyses, higher DKA prevalence in type 1 diabetes was independently associated with younger age at onset (age 5-9, odds ratio 0.57; age 10-14, OR 0.75; age 15-19, OR 0.38; all P less than .0001 compared with age 0-4), minority race (OR 1.33; P = .002), and lack of private insurance (OR 1.33; P = .010). Gender, education, and income were not significantly associated with a higher DKA, Dr. Dabelea reported.
Independent correlates of DKA in type 2 diabetes were younger age at onset (age 15-19 vs. age 10-14: OR 0.48; P = .002), minority race (OR 2.45; P = .013), and male gender (OR 0.54; P = .008).
"With changes in health care access on the horizon through health care reform, we might begin to see future decreases in DKA prevalence over time, but we believe that focused efforts to help the minority and underserved populations will be needed to achieve this goal," Dr. Dabelea concluded.
SEARCH is funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Dabelea reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: DKA prevalence for type 1 diabetes remained high at 29.8%, 28.8%, and 28.2% between 2002 and 2003, 2004 and 2005, and 2008 and 2009 (P = .43).
Data source: An analysis of 5,618 youth, aged less than 20 years, with newly diagnosed diabetes in the SEARCH for Diabetes in Youth study.
Disclosures: SEARCH is funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Dabelea reported having no relevant financial disclosures.