User login
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
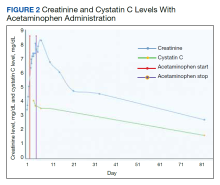
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004