User login
Acetaminophen as Renoprotective Treatment in a Patient With Severe Malaria
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
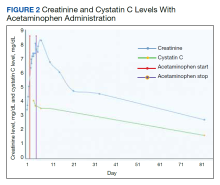
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
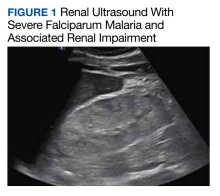
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Role of Point-of-Care Ultrasonography in the Evaluation and Management of Kidney Disease
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
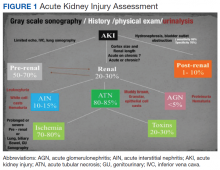
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
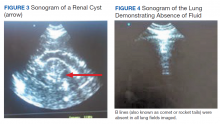
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
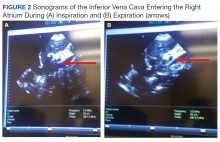
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
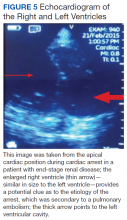
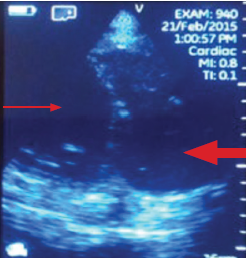
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.