User login
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
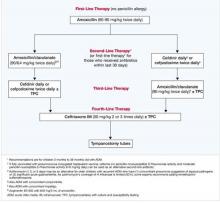
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.