User login
Anticipated Effects of Pneumococcal Vaccines on Otitis
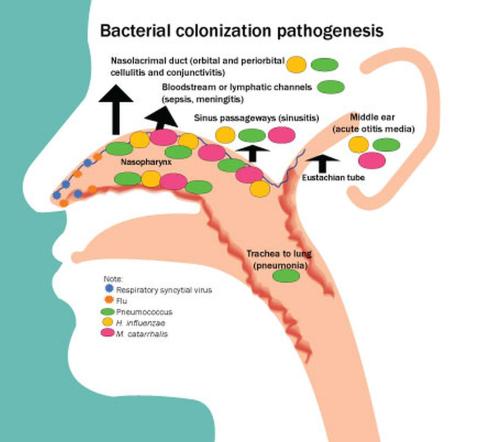
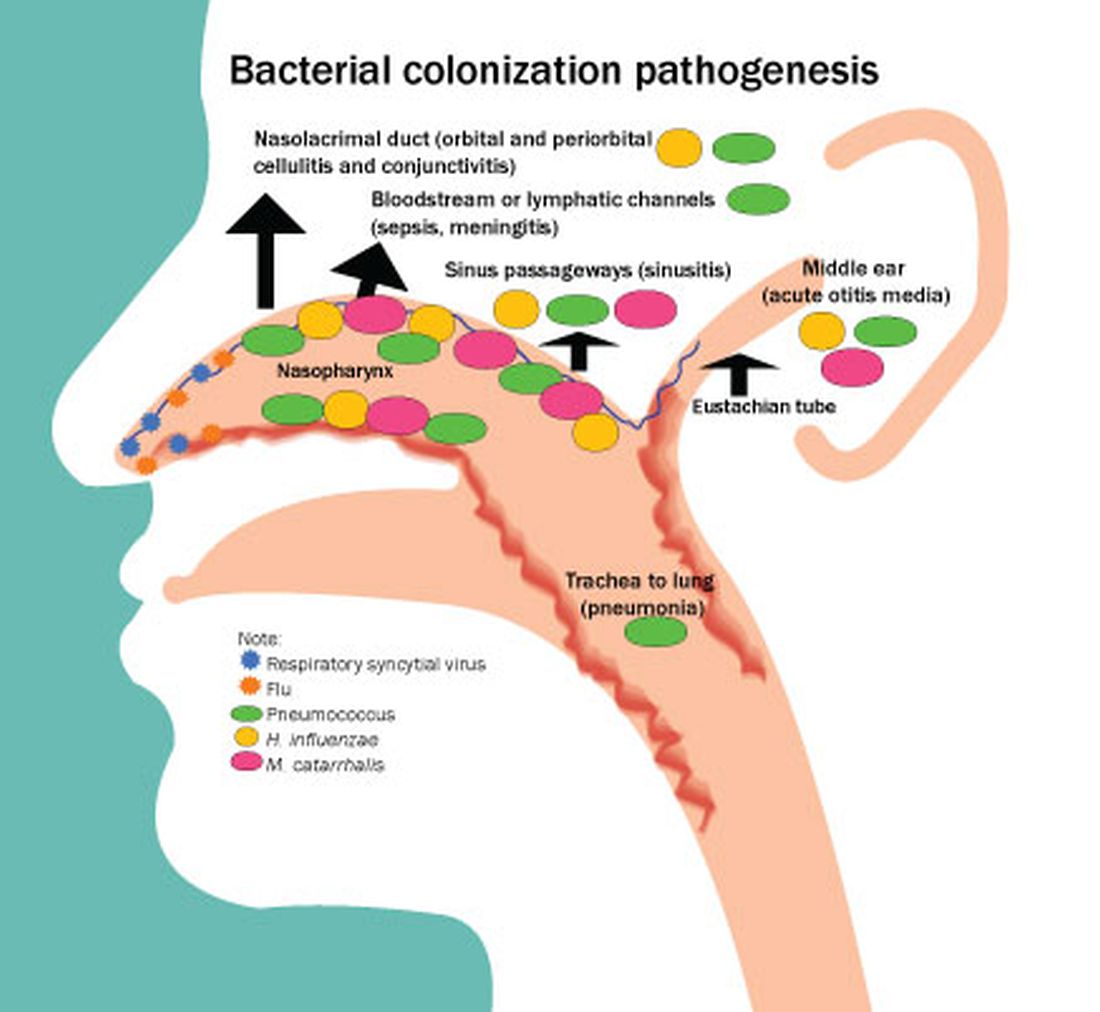
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
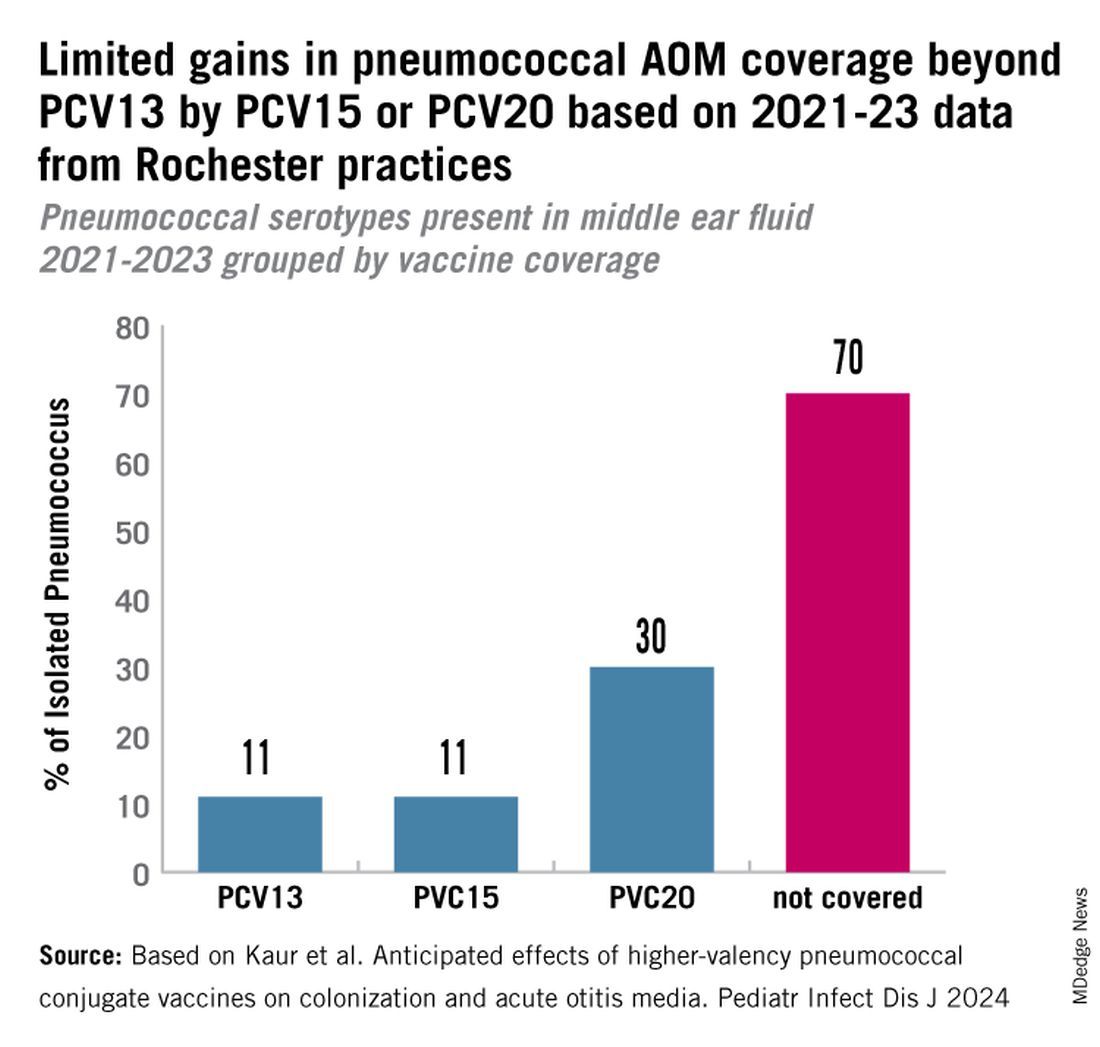
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Predicting and Understanding Vaccine Response Determinants
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
Microbiome Impacts Vaccine Responses
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
Laissez-faire
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
Profile of respiratory bacteria in children younger than 6 months
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
Young children quickly outgrow the need for ear tubes
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Acute otitis media pneumococcal disease burden in children due to serotypes not included in vaccines
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).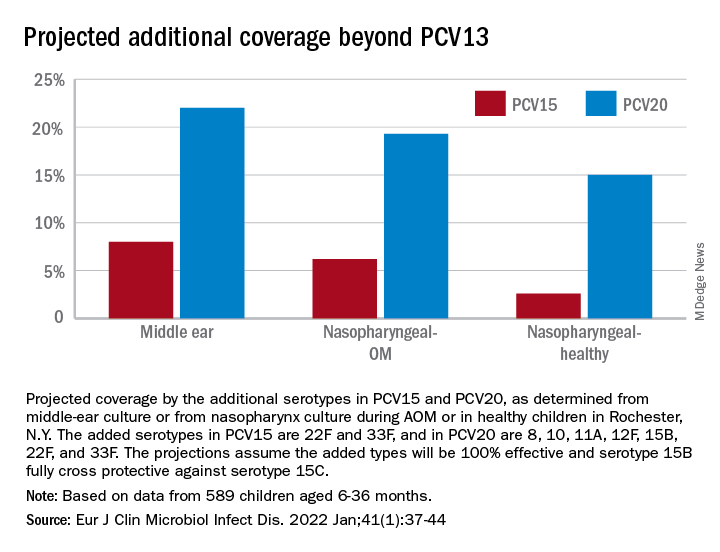
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
Antibiotics use and vaccine antibody levels
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
Effect of COVID-19 pandemic on respiratory infectious diseases in primary care practice
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.